Introduction
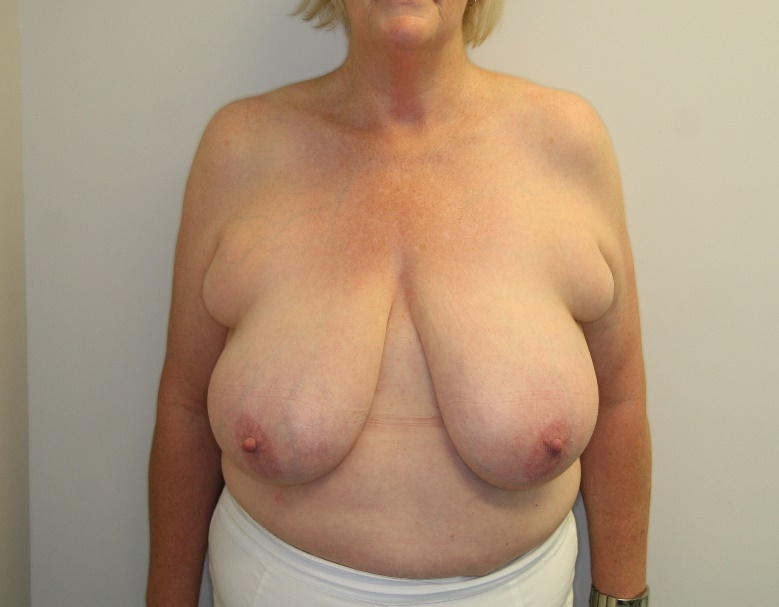
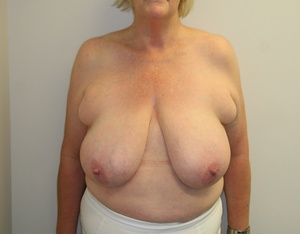
Reduction mammoplasty is a procedure undertaken primarily to reduce the volume of the breasts while maintaining the aesthetics and function of the organ.1 Women suffering from hypertrophic breasts improve their quality of life—physically, psychologically and also psychosocially—after breast reduction surgery even though complications are common and the most feared complication is necrosis of the nipple areolar complex. Large-volume breast reduction, long sternal notch to nipple distance and revisionary breast reductions all have a higher risk of postoperative nipple necrosis.2–6
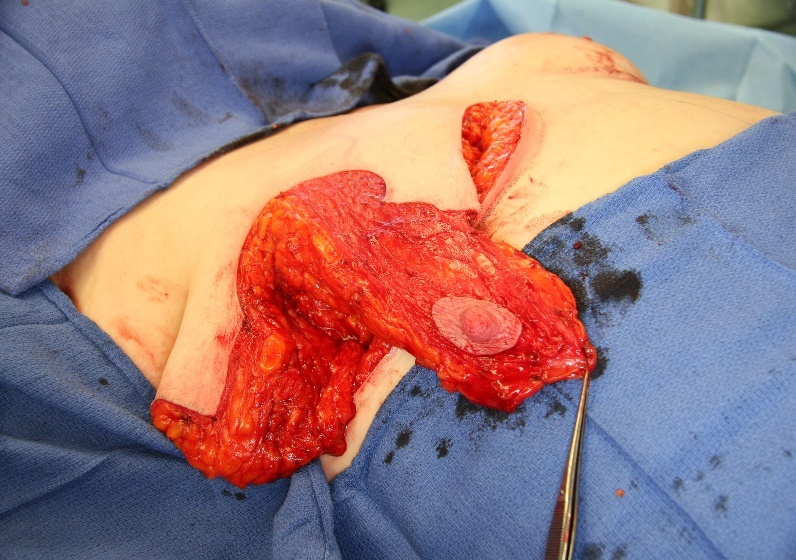
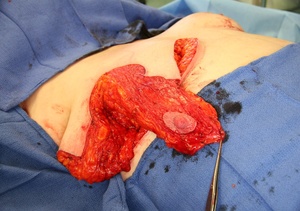
The technique of removing the nipple and replacing it as a full thickness graft is sometimes considered in these high-risk procedures.5 However, free grafting is associated with disruption of the ductal system, reduction in sensation of the nipple areolar complex (NAC) and a generally less optimal aesthetic outcome.7,8
The vascular supply to the nipple is an essential consideration in surgical planning to avoid the complication of nipple necrosis.5,9–13 Previous studies of the dominant vascular pedicle have been limited to a small number of cadaver dissections10–12 and, more recently, MRI analysis.13
The aim of the present study was firstly to investigate the vascular supply to the NAC using angiographic computerised tomography (CTA)14 and secondly to investigate if the preoperative CTA could change the surgical planning to reduce the incidence of nipple necrosis after breast reductions in patients considered to be in at a high risk of nipple loss.
Methods
Phase 1: Determination of the blood supply to the NAC
Using a dataset of all female CT thoraces performed at a single centre between January and May 2013, based on the following selection criteria (n = 132):
-
the patient was a physically developed female
-
at least one breast and potential blood supply was visible in extended fields
-
intravenous contrast enhancement was visible in the anterior chest wall and
-
prior breast surgery or patients with organic breast disease were excluded.
The image analysis was undertaken by a single cardiothoracic radiologist review using maximum intensity projection (MIP) and multiplanar reconstruction (MR) on a workstation facilitating interactive reconstruction. The arterial sources, which intercostal space that was perforated, glandular/subcutaneous course and vessel entry point into the NAC of each breast were recorded. Details of the radiological protocol are available in our original publication on this technique.14
Phase 2: Clinical use of CTA in the planning of high-risk breast reductions
Of 392 breast reductions performed by a single surgeon between 2008 and 2014, 28 patients were considered as high-risk based on the following criteria:
-
the breasts were greater than 1,000 grams per breast (preoperatively estimated) (15 patients) and/or
-
the length of the nipple to sternal notch was greater than 30 cm (11 patients) and/or
-
the patient had had revisionary breast surgery, procedure details unknown (two patients).
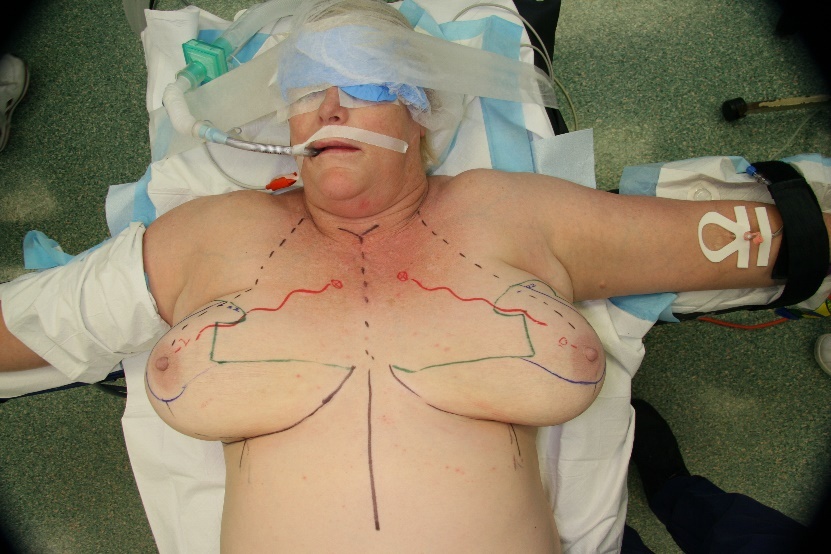
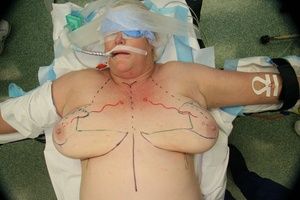
The selected patients underwent a preoperative planning CTA. The CTA images were reviewed and the anatomical information was used to determine the dominant vessel supply to the NAC.
The choice of breast reduction pedicle at the time of surgery was planned based on the anatomical knowledge from the CTA. The patients were postoperatively monitored for complications due to infection, necrosis of skin flaps and necrosis of the nipple.
Ethics
Ethics approval was granted by the St John of God Hospital, Subiaco (ref. 721), in accordance with National Health and Medical Research Council guidelines.
Results
Phase 1: Determination of the blood supply to the NAC
The analysis was performed on CTAs of 69 patient cases, involving 132 breasts. As indicated in Figure 1, the dominant blood supply was the internal mammary artery (IMA) in 96 breasts and the long thoracic artery (LTA) in 21 breasts. These results can be used to plan surgery and minimise the risk of necrosis to the NAC.
Phase 2: Clinical utilisation of CTA in the planning of high-risk breast reductions
In 27 of the 28 cases the preoperative planning was undertaken as guided by the CTA. In one case an alternative pedicle was used at the time of surgery to maintain symmetry.
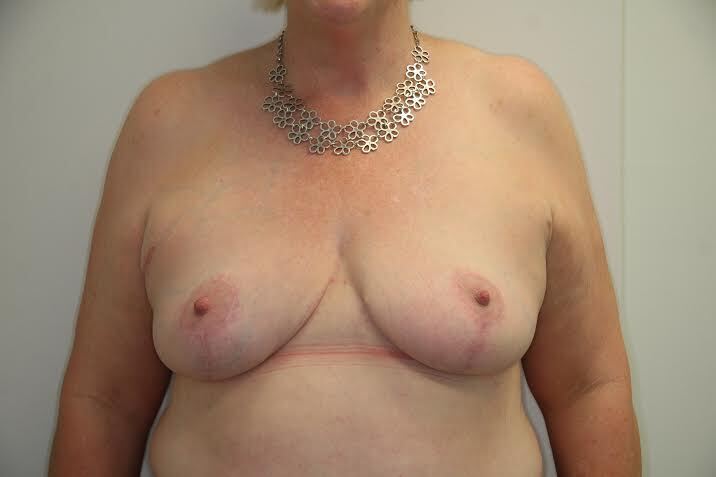
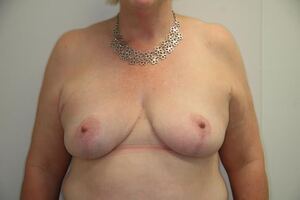
Of the 28 cases there was one case of unilateral infection, one hematoma and a single case of partial thickness areolar necrosis which healed conservatively with residual depigmentation (Table 3). No patients required free nipple grafts.
Discussion
We stress that CTA is only needed in a small percentage of the average surgeon’s breast reduction population. We feel this is a reliable alternative to a ‘cross your fingers’ approach to higher-risk patients. Surgical planning is focused on reducing risk and optimising outcomes for the patient. To be able to reduce the risk of necrosis to the NAC, knowledge of vascular anatomy is essential.3 This is especially true if the breast volume is very high and the breast is ptotic, which will result in a long pedicle, and therefore a long distance for the blood to reach the NAC.
With the rise of bariatric procedures there is an increasing number of patients requiring breast reductions following large volume weight loss.15,16 Many of these patients have long sternal notch to nipple distances placing them in the higher-risk category for developing nipple necrosis and potential nipple loss.4,5
In individuals undergoing revisionary breast reduction or breast reduction following treatment for breast cancer, the vascular anatomy will be distorted and cannot be relied upon. With the increasing globalisation of surgery it is becoming more common for patients to travel internationally for a procedure and documentation regarding the original surgical technique used is not always accessible.
Historically, these groups of patients might have been candidates for free nipple grafts, or at least having a greater risk of nipple necrosis. However, the use of a preoperative CTA allows the surgeon to proceed with confidence in relation to pedicle design and potentially reduce the risk of necrosis of the NAC while also eliminating the need for free nipple grafting where there is insufficient intraoperative blood to the NAC.
The level of patient exposure to ionizing radiation of the CTAs performed in the study was 3–6mSv. This is the same or less than the average annual background radiation exposure and equates to 2mSv.14 Therefore we consider the possibility of avoiding nipple necrosis in higher-risk breast reductions outweighs the problems associated to the limited exposure of radiation during the CTA.
As the dominant blood-supply to the NAC is identified, the surgeon can individualise the surgical planning of the direction of the pedicle. It is important to note that the dominant blood supply to the NAC is not always symmetrical; three of the patients in the clinical case series had asymmetrical dominant blood supply to the NAC. The question of a symmetrical approach is then raised. Interestingly, the one case with the complication of partial thickness areolar necrosis was the single case where the pedicle design was not based on the dominant vessel demonstrated on CTA. The patient in question had an asymmetrical NAC blood supply, IMA (right) and LTA (left). A bilateral superomedial pedicle design was used, despite the CTA result. The left breast developed partial thickness areolar necrosis postoperatively which healed with conservative management.
The complications reported in this series of cases are in line with previously reported lower-risk breast reductions.3 In these cases, NAC integrity has been maintained in a group of women considered to be at higher risk of necrosis of the NAC.
The weakness in this study is the lack of randomisation which means that we cannot be sure that the result would have been the same if another pedicle was chosen. The small numbers in the clinical series reflect the relative rarity of these higher-risk cases. However, the single case that did have partial necrosis to the nipple could indicate that it might be better to plan surgery and the type of pedicle based on the dominant vessel to the NAC. The number of patients is also small which can, by chance, give the low incidence of complications seen in this study.
Conclusion
Preoperative CTA for high-risk breast reduction may be a useful surgical planning tool. With the insight gleaned from the CTA, pedicle design could be tailored to the individual’s anatomy. This surgical planning may reduce the incidence of necrosis to the NAC in the women who have a higher risk of nipple areolar necrosis.
Acknowledgements
The content of this article was presented at the PSC in Brisbane, Queensland, 6-10 May 2015.
Consent to publish
Patients signed informed consent regarding publishing their data and photographs.
Disclosure
The authors have no conflicts of interest to disclose.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: 15 January 2018 AEST
.png)

.jpeg)
.png)

.jpeg)