Introduction
Mandibular reconstruction following traumatic injury or oncological resection poses unique challenges to the surgeon, secondary to the complex and variable anatomical space. This challenge is accompanied by the goal to restore a degree of postoperative symmetry and functional mandibular movement that will ultimately impact quality and longevity of life.1 Historically, the bony free flap used to reconstruct the mandibular defect, commonly harvested from the fibula, was taken using a freehand technique. The flap was then fixed in place by titanium plating that was bent intraoperatively to the contour of the mandible.1 This technique required a trial and error approach that compromised reconstruction accuracy and added complexity to an innately complex procedure.2,3 The field was revolutionised by the introduction of virtual surgical planning (VSP) that used 3D segmentations from patient computer tomography (CT) scans to design individualised osteotomy guides. This has resulted in improved accuracy and mandibular symmetry, reduced time in the operating theatre and reduced cost.3–5
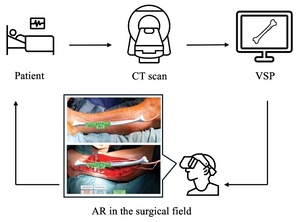
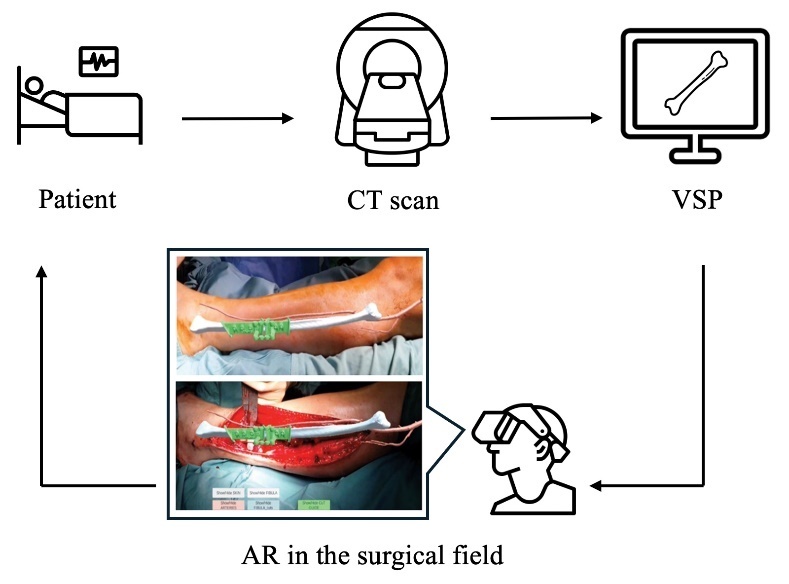
Although VSP is now gold standard, it exhibits several problems. The preparatory time to print a 3D guide increases the turnaround time for preoperative planning, which may not be suitable for trauma or other emergency cases.6 Additionally, this method requires the surgeon to either have access to an in-house 3D printer or outsource this capability. Either option incurs significant costs that neutralise the economic advantage gained by reduced intraoperative time. The 3D printed (3DP) cutting guide demands direct contact with bone and complete alignment may not be achieved in the presence of surrounding fascia and muscle, introducing deviation from the planned osteotomy.6 Recently, the use of augmented reality (AR) and mixed reality (MR) is being explored as a potential solution to the aforementioned downsides of 3DP guides. Augmented reality involves merging virtual images with the physical environment, while MR additionally facilitates interaction with these images.7 The ability to view preoperatively developed images in the surgical field in real time, such as osteotomy lines and anatomical models, could be the next front of exploration in mandibular reconstruction. In theory, the absence of hardware in the surgical field coupled with no requirement for 3D printing could reduce pre- and intraoperative time, as well as procedural costs.8 Additionally, AR/MR could delineate anatomy otherwise not visible to the surgeon, such as vasculature and bony landmarks.9 Figure 1 displays the general AR workflow and an application in mandibular reconstruction.
This review aims to systematically evaluate current applications and future feasibility of AR/MR technology in mandibular reconstruction surgery. This is investigated primarily in the context of system workflow and accuracy. Secondary outcomes include user experience, specifically system useability, functionality and ergonomics. Other secondary outcomes investigated include complications, time and procedural costs. The review also identifies current limitations and future directions of this emerging field.
Methods
Study design
This review was conducted within the current guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) framework, and was guided by the synthesis without meta-analysis in systematic reviews (SWiM) framework.10,11 The review protocol was registered with the PROSPERO systematic review database (PROSPERO 2023 CRD42023395665).
Eligibility criteria
Primary research that investigated clinical application of AR or MR technology in mandibular reconstructive surgery was eligible for inclusion. Augmented reality and MR were generally defined as wearable and non-wearable technology that overlays and/or incorporates data and virtual images into the physical environment. For inclusion, studies were required to report at least one of the outcomes of interest (as stated above). There were no restrictions on level of evidence for this review or subpopulation. Omitting studies of lower evidence such as case studies may have risked omitting significant developments in this emerging field. Articles available in English were included in this review.
Studies investigating other craniofacial procedures were excluded from this review. Papers exploring the use of virtual reality exclusively were excluded. Studies published prior to the last 15 years were excluded, unless exhibiting seminal findings to the field. The timeframe was chosen to account for technological evolution, excluding outdated technological modalities. This approach aimed to maximise the quality and currency of the evidence included in the review. Papers investigating AR/MR exclusively in an educational setting were excluded.
Search strategy
The primary literature search was conducted using the MEDLINE database on PubMed and the Embase database in February and March 2023. The extensive search strings were devised using the PICO (patient, intervention, comparison, outcome) framework (Supplementary material 1). Reviews were not considered for inclusion. However, citation searching was employed to screen for relevant primary studies. A manual search of ‘Augmented reality in mandibular reconstruction’ was conducted, screening the first three pages of Google Scholar Database to identify additional literature.
Selection process and data collection
One author was involved in screening titles and abstracts with an additional author for full text screening. Both authors screened independently before comparing results. Using the eligibility criteria as a reference, disagreements were resolved through discussion when a consensus on the language definitions and terminology was made. Endnote was used to store references throughout this process.
One author collected data from individual studies using a form in Microsoft Excel (version 16.92, Microsoft Corporation, Redmond, Washington, USA). A second author reviewed the form designed for data extraction to ensure the relevance and appropriateness of information drawn from extraction. Data was extracted in relation to study characteristics, study design, population, intervention and outcomes. All results relevant to each outcome domain were sought throughout this process.
Risk of bias
Tools were applied for quality assessment of the variety of study designs included in this review. For cohort and case control studies, the Newcastle–Ottawa scale was applied.12 Case series and case reports were subjected to the methodological quality and synthesis of case series and case reports quality assessment tool.13
Heterogeneity
As this review was not amenable to meta-analysis, heterogeneity was explored in the clinical and methodological context only. A statement on clinical and methodological heterogeneity can be seen in the results, guided by the updated edition of the Cochrane Handbook for Systematic Reviews of Interventions.14
Results
Study selection
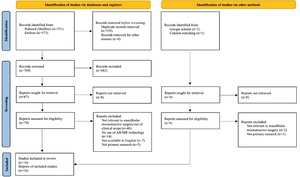
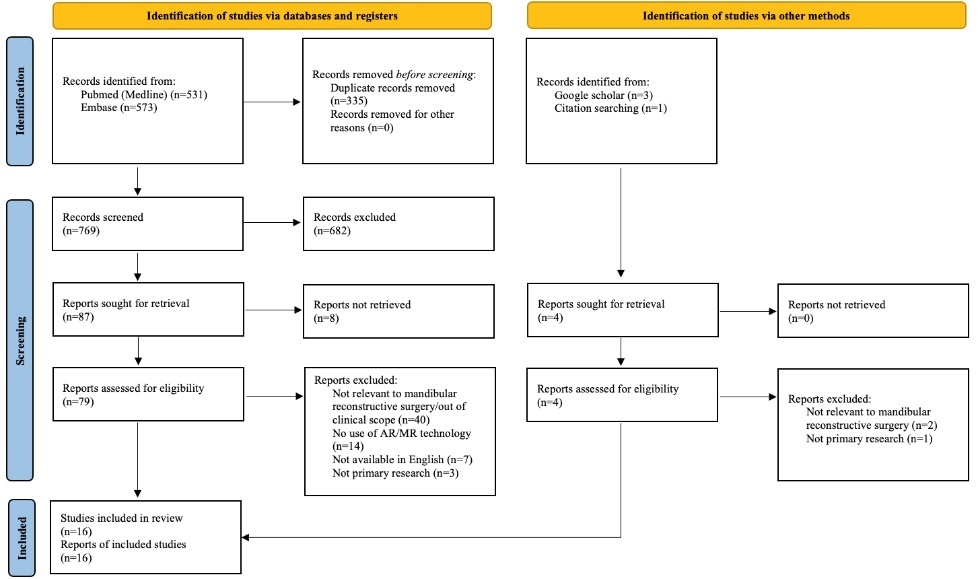
The primary literature search yielded 769 results after duplicates were removed. Following abstract and full text screening, 16 papers were included in the final review (Figure 2).
Study characteristics
Study characteristics are summarised in Table 1. Three studies were case reports,15–17 six were case series,9,18–22 and seven were cohort studies.7,23–28 Publication date ranged from 2012 to 2022. Subpopulations varied and multiple articles described more than one. Ten studies involved human participants,9,15–20,22,23,26 three studies involved human cadavers,7,24,25 one study was conducted on an animal cadaver,21 and six studies used phantom models.17,21,25–28 Human patients were the primary subpopulation in case reports and case series.
Risk of bias in studies
Risk of bias/quality assessment was quantified with a score from the relevant tool. These scores are displayed in Table 1. Cohort studies scored either 6/9 or 7/9 on the Newcastle–Ottawa scale.12 Case series and case reports scored between 3/5 and 5/5 using the methodological quality and synthesis tool.13
Outcomes
Workflow
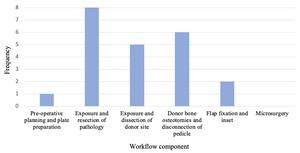
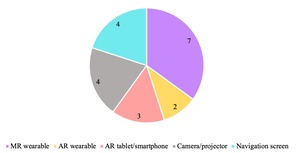
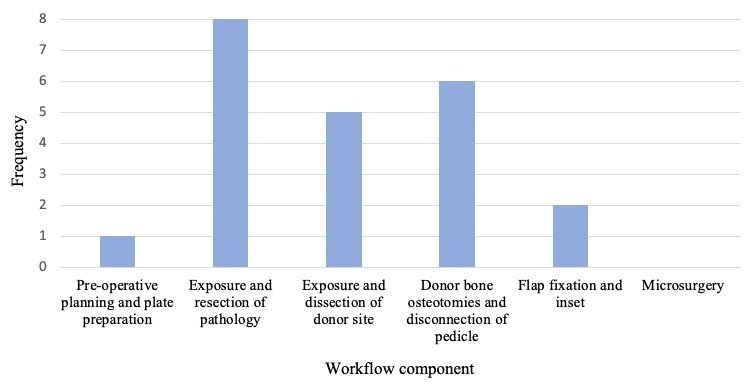
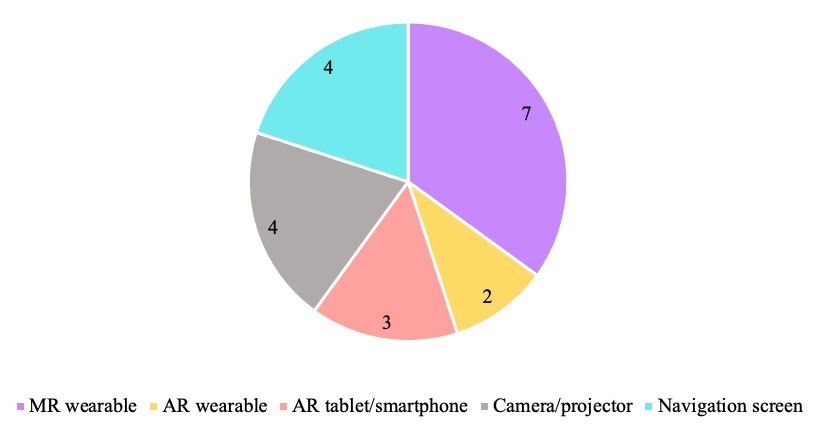
Workflows of individual studies were grouped to identify the main steps of mandibular reconstruction that applied AR/MR technology, seen in Figure 3. Specific AR/MR systems varied between studies and some studies described multiple systems. These include wearable MR headset,7,15,16,18,20,23,26 a camera or projector,19,21,22,24 a navigation screen,17,25,27,28 AR applications on smart phone/tablet,9,19,26 and AR eyewear27,28 (Figure 4). All studies using MR headsets,7,15,16,18,20,23,26 AR applications on smart phone/tablet,9,19,26 and AR eyewear27,28 were published within five years of this review.
Yodrabum and colleagues19 used AR for cutaneous perforator mapping at the donor site and in planning cutting guide placement preoperatively. This was the only application at this stage of the procedure seen in the literature (Figure 3). No studies used the technology to inform preoperative plate preparation.
Eight studies applied AR/MR during exposure and resection of the pathology (Figure 3).16–18,20–23,28 Articles described the use of AR/MR in overlaying relevant structures including mandibular bony anatomy,16,18,20,22 the path of the inferior alveolar nerve,21 and visualisation of tumour borders.16,22 Other applications included displaying the VSP in the surgical field to assist mandibular osteotomy and tumour resection,18,20,22,23,28 and to provide live feedback of oscillating saw angulation.17
Five studies utilised AR/MR in donor site exposure and dissection (Figure 3).9,15,16,19,26 This included superimposition of fibula anatomy,9,16 skin paddle tracing26 and preoperative cutaneous mapping to assist dissection.19 Lin and colleagues15 used wearable MR to simulate donor site incision and fibula osteotomy in the surgical field.
Six studies used AR/MR in osteotomy of donor bone (Figure 3).7,9,16,24,25,27 This involved osteotomy plan overlay at the surgical site,7,16,24,27 or on a navigation screen.25 Battaglia and colleagues9 used wearable MR to inform fibula cutting guide placement.
Two studies applied AR/MR systems in the flap fixation and inset component (Figure 3). Suenaga and colleagues17 displayed drill trajectory for mandibular drilling tasks on a navigation screen in real time. Yang and colleagues20 used MR to reference the ideal reconstructed mandible in the surgical field during flap shaping and inset. No studies described an application in microsurgery for vascular anastomosis.
Accuracy
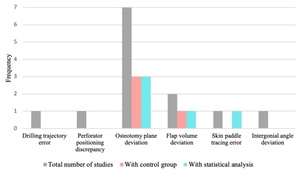
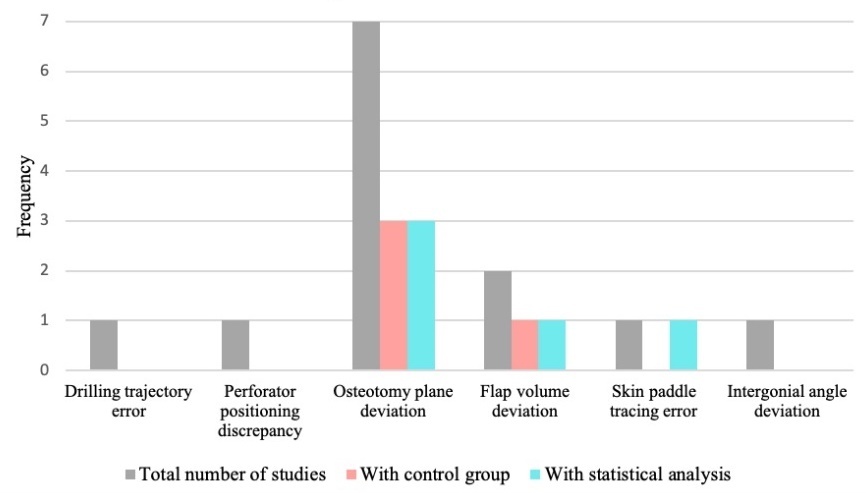
Accuracy was an outcome measure in 10 studies.7,17–19,23–28 Table 2 highlights the task and corresponding accuracy outcome assessed, a summary of findings, and statistical analysis where applicable. Figure 5 depicts accuracy measures across studies, and the frequency of those employing control groups and statistical analysis. Multiple studies involved more than one measure.
Mandible or bony flap osteotomy deviation from ideal reconstruction was the most common accuracy measure, seen in seven studies (Figure 5).7,18,23–25,27,28 This was measured predominantly via superimposing post-intervention CT scans onto the VSP, detecting plane deviations from the planned reconstruction.7,18,23–25,27,28 Calipers were used to measure plane distance in one study.18 Three studies included a control group (3DP guides) and performed statistical analysis, as seen in Figure 5.24,27,28 These studies observed reduced angular deviation from the VSP using 3DP guides versus the AR/MR intervention in at least one plane.24,27,28 Regarding plane distance, results were mixed. Two studies demonstrated no significant difference in deviation between 3DP guides and AR/MR,24,27 while another identified significant deviation from specific points using AR compared to guides.28 The remaining measurements reported without statistical analysis or control groups can be seen in Table 2.
Flap volume deviation/overlap from VSP was the second most frequently reported measure, assessed in two studies (Figure 5).24,25 One study revealed no significant difference in iliac flap volume when using AR and 3DP guides.24 Zhao and colleagues25 reported over 95 per cent overlap of mandible reconstruction against ideal reconstruction. This study did not use a control or statistical analysis.
Skin paddle tracing using an AR tablet and an MR wearable was assessed against a template by Cercenelli and colleagues.26 The MR device was found to have greater tracing success than the AR tablet at the 0.5 mm template accuracy level, with no significant difference at the higher accuracy templates.26
Other accuracy measures identified in this review included MR-assisted drilling trajectory,17 perforator positioning discrepancy using AR against a Doppler ultrasound,19 and intergonial angle deviation at the mandible.7 No statistical analysis or control group was used with these outcome measures (Figure 5). Outcome measures can be seen in Table 2.
Secondary outcomes
Useability, functionality and ergonomics were not formally assessed, however they were reported in 10 studies.7,9,15,19–22,26–28 Regarding useability and functionality, general disadvantages included low image resolution,15 suboptimal brightness and transparency,7,26 and poor depth perception.22 Other papers described noticeable image deviation with head movement.15,20 Advantages reported included being able to visualise anatomy in the surgical field with AR,22 and its usefulness in combination with cutting guides.9 Using a screen was beneficial as it allowed visualisation by the team,21 and AR eyewear was reported to improve coordination and orientation in the surgical space.28 One study reported that AR devices improved communication between team members.19
Regarding system ergonomics, disadvantages included that the MR device was reportedly cumbersome to wear15 while the AR tablet proved difficult to hold.19 Other authors described favourable ergonomics with the MR wearable device26 and the benefit of lightweight AR eyewear.27,28
Other secondary outcomes of this review included time, cost and complications. Tang and colleagues23 observed no complications intraoperatively. Three studies implemented follow up postoperatively, with no reported complications.15,20,23 Two studies stated that intraoperative time was within acceptable limits,9,18 with one of the articles noting no negative impact from the use of the technology.9 No further elaboration was offered. A third article reported the total time to prepare the AR system preoperatively was three hours.26 Cost was not evaluated by any of the included studies.
Heterogeneity
Overall, this review exhibited high clinical heterogeneity secondary to diversity in subpopulations, interventions and outcome measures. Subpopulations varied from human participants to cadaveric specimens and phantom models (Table 1). Augmented reality and MR systems and applications in workflow varied dramatically, as previously discussed. Finally, accuracy measurements were highly variable between studies, further contributing to clinical heterogeneity (Table 2).
The review exhibited moderate methodological heterogeneity, due primarily to differing study designs. Study design included case reports, case series and prospective/retrospective cohort studies. Differing in risk of bias assessment can also contribute to methodological heterogeneity. However, cohort studies only varied by one point on the Newcastle–Ottawa scale.12 Case reports/case series varied by two points using the methodological quality and synthesis tool.13 This reflects a somewhat consistent and acceptable risk of bias across studies.
Discussion
To the authors’ knowledge, this is the first comprehensive review investigating the current application and feasibility of AR/MR technology in mandibular reconstruction with autologous bone.
Workflow
The overall process of dissecting, exposing and harvesting the donor flap in combination was described in 11 instances, and this is the most common AR/MR application in the literature (Figure 3). This included delineating fibula bony anatomy9,16 and assisting in cutaneous mapping, such as displaying skin paddle design on the surface prior to incision.19,26 This functionality is not available with 3DP guides. Augmented reality and MR systems have also been used to assist in donor bone osteotomy, as an alternative to a 3DP guide7,16,24,25,27 or to inform guide placement.9
Similarly, numerous applications were observed in exposure and resection of mandibular pathology. This was predominantly used to display relevant anatomy,16,18,20–22 delineate tumour borders,16,22 and assist in osteotomy via intraoperative access to the VSP.18,20,22,23,28 Although useful, delineating tumour borders and anatomical landmarks relies heavily on accurate imaging, due to evolving margins and tumour infiltration. Bone marrow infiltration and perineural invasion must also be considered which will be largely undetectable using imaging.
This review also identifies aspects of the procedure that used AR/MR less frequently. The technology has not been described in preoperative mandibular plate preparation and microsurgery. Visual data may not be sufficient without the tactile feedback that a 3D printed model will provide during plate bending. Furthermore, the challenge of magnification and additional optics may deem AR/MR unfeasible in microsurgery and has not been explored likely for that reason. If this is the case, the same challenge would also be seen in perforator and pedicle dissection when raising the flap under magnification.
Wearable MR devices were the most frequently employed system (Figure 4) and involve a head worn device superimposing images onto objects in the field of vision.7,15,16,18,20,23,26 Additionally, the wearable devices were exclusively described in more recent studies, within five years of this review, indicating a developing interest in this technology over non-wearable systems. This could inform a focus on wearable MR devices in further research and potential future clinical application.
Overall, these findings demonstrate that future developments in wearable MR technology may be best directed towards aspects of harvesting the flap, such as initial dissection and osteotomy, as well as resecting the mandibular pathology, informed by common applications currently described.
Accuracy
Accuracy was an outcome in 10 studies, and was defined using six different measures overall.7,17–19,23–28 The variability of measures highlights the lack of standard reporting, with only four of those measures involving control groups,24,27,28 and half of all measures employing statistical analysis (Figure 5).24,26–28 The most frequent accuracy measure involved osteotomy plane angulation and distance deviation from the planned reconstruction.7,18,23–25,27,28 Most studies measured this using CT scan superimposition.7,18,23–25,27,28 In future, measures used to determine accuracy of osteotomy should continue to incorporate plane angulation and distance deviation from the VSP via CT superimposition. This will begin to create replicable accuracy evaluation as measurement variability is minimised.
Accuracy of the technology can be distilled into the following main findings. Augmented reality and MR technology was less accurate in osteotomy plane angulation,24,27,28 particularly in the sagittal plane,28,29 than the current gold standard of VSP. This may partly be explained by the assistance the template provides in guiding the saw through the bone. Sagittal plane osteotomy is unique to the fibula free flap harvest (there is no sagittal cut in iliac bone harvesting) and inaccuracy without a guide may be due to the disruption of line of sight by the hand. In osteotomy plane distance, results are mixed,24,27,28 while regarding flap volume, AR/MR demonstrated comparable accuracy.24,25 The overall paucity of statistical testing and diverse outcome measures does not allow further interpretation and emphasises the need for further evaluation before considering this technology in a clinical setting.
Lack of standardised reporting is not unique to AR/MR literature. A previous systematic review investigating 3DP guides in mandibular reconstruction made similar observations surrounding variable and non-standardised accuracy measures.29 Additionally, the literature does not indicate the clinical significance of inaccuracy. This could be secondary to the complexity of defining clinical significance, which would involve assessment of further follow up and patient satisfaction, as well as an accepted definition of adequate mandibular occlusion.
Secondary outcomes
Useability, functionality and ergonomics were not formally assessed in the included studies. However, key advantages and disadvantages can be summarised, serving to inform future development. Disadvantages reported include poor resolution and transparency of displayed data,7,15,26 as well as poor depth perception and image drift.15,20,22 Multiple advantages reported include displaying key anatomy in the field,22 improved coordination and orientation in the surgical space,28 and improved team visualisation21 and communication.19 Overall there remains a paucity of formal data pertaining to user experience, and a lack of tools such as surveys or structured interviews to analyse user experience. Nonetheless, advantages observed warrant further development of AR/MR, with a focus on mitigating current technological limitations.
Only four studies commented on complications, of which none were observed either intra- or postoperatively.15,18,20,23 Preliminarily this shows early promise for AR/MR safety in the clinical setting, however it requires more rigorous investigation in future research. Time has not been formally assessed, however overall no negative impact on intraoperative time was identified.9,18 One paper observed a preparation time of three hours to segment the CT scan and port the plan to the AR system.26 Although there is minimal data on print time for guides in mandibular reconstructive surgery, literature indicates that printing of anatomical models can vary from 5–16 hours, depending on the level of detail and size.30 These preliminary findings therefore demand further comparison into AR/MR and 3DP guide preparation time. There was no comment on cost in the current literature. Clinically, this is an essential factor that must be considered prior to implementation of any such prospective health intervention.
Regarding the clinical regulatory landscape, readers should recall that AR/MR technology is a medical device. Quality management and compliance with regulatory frameworks are key considerations in successful device implementation in the clinical setting.31 This ensures safe, effective and informed advances in surgical technology. Therefore, while cases using AR/MR in mandibular reconstruction have been described, the specific regulatory environment must be consulted before considering the future abandonment of 3DP guides in favour of this technology.
Limitations
Heterogeneous subpopulations imposed a substantial limitation in this review. Preclinical testing on phantom models and cadaveric specimens reduced the external validity of findings, and many studies only focused on narrow applications in the overall workflow. Furthermore, absence of standardised and validated accuracy measures limited interpretation and disallowed grouping of results. These limitations rendered a meta-analysis unfavourable. Another limitation is observed in the high proportion of study designs in the current literature devoid of control groups and statistical testing. As demonstrated, outcomes such as user experience, complications, cost and time have not been thoroughly investigated and are important considerations when surrounding clinical use. These measures directly impact the operator, the individual patient, the workflow of the department and the economic viability of the system. One of the exclusion criteria included the use of this technology in teaching, however its capabilities noted in this review justify exploration of its potential suitability in the educational setting.
Conclusion
Augmented reality and MR technology requires further research to comprehensively evaluate feasibility. This review has identified interesting applications in mandibular reconstructive surgery with autologous bone. However, current accuracy findings are variable and inconclusive, and further evaluation is required before considering use in clinical practice. Early strengths and weaknesses have been identified in useability, functionality and ergonomics that will inform future development. Furthermore, future research regarding outcomes including complications, time and costs will assist more holistic evaluation for use in the clinical setting. Overall, AR/MR is not currently a suitable alternative to 3DP guides. However further consideration and research is required to determine its potential as a tool in mandibular reconstruction.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship and/or publication of this article.
Revised: May 6, 2024 AEST; June 6, 2024 AEST