Introduction
With increased pressure on health care budgets, we are seeing more focus on the clinical and cost effectiveness of interventions provided by health systems globally. The ‘Choosing Wisely’ campaign recently adopted in a number of countries, including Australia, focuses on encouraging patients and clinicians to ‘discuss inappropriate and potentially harmful tests, treatments and procedures.’1 While the Australian campaign is seeing the development and identification of procedures that may be harmful to patients, there is a paucity of robust clinical studies that clearly define the benefit of cosmetic breast augmentation in non-pathological female breasts. Despite this, the number of cosmetic breast augmentations performed in Australia each year is increasing rapidly, with numbers of procedures more than doubling over the past decade according to the most recently documented Australian Institute of Health and Welfare (AIHW) statistics.2 In addition, there is an increasing popularity of ‘cosmetic tourism’ by Australians for such procedures.
The process of having cosmetic breast augmentation surgery in Australia involves a completely private arrangement (and transactions) between a surgeon, anaesthetist, hospital and patient, with no funding or rebates available through the Medicare-based public health system. The procedure is not limited to Royal Australasian College of Surgeons (RACS) accredited surgeons and, in many instances, the doctor performing the surgery is a cosmetic proceduralist. Further, the Australian Society of Plastic Surgeons’ Breast Implant Registry does not capture data related to non-specialist surgeons who perform the procedures.
The most commonly documented complications that arise from cosmetic breast augmentation surgery are capsular contracture, implant rupture, infection, seroma formation, haematoma, deflation, mal-positioning and/or rotation of the implant, nipple and breast pain or sensation changes.3–13 While the severity and implications of complications vary widely according to individuals’ circumstances, they often require further outpatient and inpatient surgical treatment.
Government subsidies cover part or all of the surgical and medical costs associated with the subsequent treatment of complications, even though this ‘burden of disease’ is created entirely from non-essential cosmetic operations. The management of these complications therefore places some level of burden on the health care system. Hanefeld and colleagues14 suggested that the economic burden to the United Kingdom’s National Health Service (NHS) from complications arising from cosmetic tourism was £8.2 million per annum.
Methods
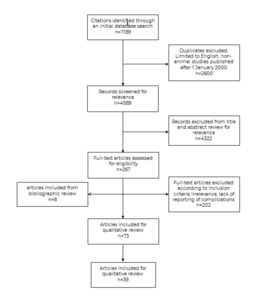
Using the PRISMA 2009 statement we conducted a systematic review to find articles reporting complication rates following cosmetic breast augmentation using breast implants (Figure 1).
A literature review of PubMed, Ovid Medline and the Cochrane (Central) database was performed to identify relevant articles using the following search terms: ‘breast augmentation’, ‘cosmetic breast surgery’, ‘breast implants’, ‘breast prosthesis’, ‘augmentation mammoplasty’, and ‘cosmetic breast augmentation’. Additional terms included ‘complication’, ‘revision’, ‘capsular contracture’, ‘cost’, and ‘explantation’.
The inclusion criteria were:
-
English language
-
explicit documentation of complication rates following cosmetic breast augmentation
-
publication later than 1 January 2000
-
mean follow-up greater than one year.
Exclusion criteria were:
-
studies involving Poly Implant Prothese (PIP) implants
-
studies published prior to 1 January 2000
-
mean follow-up less than one year
-
revision augmentations and cosmetic augmentation performed overseas.
No restrictions were placed on the type of article, however, special consideration was needed for statistical analyses appearing in multiple publications arising from the same long-term studies. In this circumstance, duplication was avoided by including only the most recent publication regarding the study.
Data extracted from each study included author, year of publication, level of evidence, type of study, number of patients, follow up, type of prosthesis used and indication, and outcomes measured. We extracted data regarding all reported complications, however, analysis was performed only on more common short-term complications involving infection, haematoma and seroma and long-term complications involving capsular contracture, rupture and re-operation rates. We attempted to report complications on a ‘per-patient’ basis rather that ‘per-implant’. Articles that reported complications by implant were converted to a figure according to their overall study population to allow analysis. Our figures may therefore show slight discrepancies when compared with original published data. Incidence of breast augmentation procedures was extracted from publicly available data from AIHW.2
Using a top-down costing approach, we calculated the direct cost of surgical fees for complications. We then modelled the cost over specific periods for re-operations arising from breast augmentation. Our analysis required specific population and surgical assumptions to enable the calculations, including:
-
that all procedures performed are primary augmentations (so as to not over-report complication rates if we over-estimated the rate of revision augmentations)
-
the ratio of saline to silicone implants in Australia is 5 per cent saline and 95 per cent silicone (to prevent over-estimation of higher complication rates associated with saline implant data)
-
complication rates identified by current literature worldwide apply to the Australian population.
Operations required for the treatment of complications or for revision following breast augmentation were matched to appropriate Medicare procedure codes including:
-
exchange of implant procedures (with or without capsulectomy)
-
explantation of prosthesis
-
mastopexy
-
other miscellaneous operations such as drainage of haematoma or seroma, or debridement of infected surgical wound.
Procedure codes used in our cost analysis are documented in Appendix 1 with the overall Medicare benefit attributable to each (including surgeon and assistant fees). For cost analysis we modelled the ratio of procedure codes proportionally for each cause of re-operation. The ratios reflect the common reasons for re-operation and an approximate rate that these occur. Our model is designed to reflect the complications according to the proportions demonstrated in the literature.3–12
Results
The literature search was conducted in September 2017. The search and selection process is summarised in Figure 1. Of the 267 articles assessed for eligibility, 73 matched inclusion criteria and were reviewed. Of these, 39 studies sufficiently reported outcome data suitable for inclusion in a quantitative analysis of complication rates. Appendix 2 provides details of the 39 studies used in the quantitative analysis.
On the reported complications arising from 39,738 breast augmentation patients, meta-analysis revealed:
-
2462 primary saline augmentations (six studies)
-
32,838 primary silicone augmentations (31 studies)
-
4438 revision silicone augmentations (11 studies).
Where implant type or indication was not explicitly reported, studies were excluded. Tables 1–3 show the complication rates identified. No articles reporting on complications specific to revision augmentation with saline prostheses met the criteria for inclusion in the analysis.
Primary augmentation with saline prostheses
Only six articles reported the outcomes of saline prostheses following primary augmentation. Table 1 summarises rates of complications reported in each study.
Infection, seroma and haematoma complications were poorly reported in these studies. Only one study reported haematoma rates in a study of 20 patients.15 No studies reported seroma rates, and two studies reported infection rates.15,16 The overall capsular contracture rate was 15.6 per cent (10-year rate 21.6%). Rupture (or ‘deflation’) was reported in three studies at an overall rate of six per cent (10-year rate 7.4%).15–17 Re-operation occurred in 31.8 per cent of the 1793 cases (10-year rate 44.1%).
Swanson and colleagues18 reported re-operation rates of 10.7 per cent for augmentation alone and 20.5 per cent for augmentation with mastopexy based on average follow-up periods of 8.3 months and 8.4 months respectively. Given the follow-up periods in this study were shorter than 12 months, it was not included for quantitative analysis.
In five-year reported complication rates by Walker and colleagues,17 other significant complications such as asymmetry, breast pain and malposition were noted in 12.2 per cent, 17.0 per cent and 9.2 per cent of patients respectively. Swanson and colleagues18 reported asymmetry in 3.8 per cent and 3.4 per cent respectively in the two groups described above. A significant positive correlation was detected between the incidence of complications and patient age but the correlation was weak (r = 0.10, p < 0.01). Swanson and colleagues also showed that smoking was associated with increased complication incidence, as was revision breast surgery (p < 0.01).
Primary augmentation with silicone prostheses
Thirty-one articles report on outcomes following primary breast augmentation with silicone implants and met the criteria for inclusion in the quantitative analysis. Significant Food and Drug Administration (FDA) pre-approval studies following a 1992 moratorium on silicone implants in the USA provide most of the long-term data for these types of prostheses. Core studies included in this analysis6,7,9 provide prospective, long-term analysis of silicone implants and associated complications. Table 1 shows the categorical data for each included study.
The 31 articles showed an overall re-operation rate of 11.4 per cent (10-year rate 23.8%) following primary augmentation with silicone. This is significantly lower than reported rates from all of the FDA studies conducted prospectively over 10 years.6,7,9 Araco and colleagues19 report a comparatively very low rate of re-operation in one of the largest cohorts included in this analysis involving 3,002 patients. Caplin,9 Maxwell6 and Spear7 all report re-operation rates greater than 25 per cent at 10 years.
Asymmetry and rippling were commonly reported complications not analysed for overall occurrence. Reported rates of asymmetry ranged from 0.8 per cent to seven per cent, while malposition was reported in as many as 5.3 per cent of cases (range 0.8 to 5.3%). Rippling was sparsely reported, but documented rates were low (range 0.7 to 2.0%). Other complications such as nipple sensation change, breast pain and palpable implant edges were less commonly reported.
Revision augmentation with silicone prostheses
Eleven articles reporting on outcomes following revision augmentations using silicone prostheses were used in the quantitative analysis. Seven of these articles prospectively studied the outcomes of patients undergoing revision augmentation. Indications for revision augmentation are sporadically reported throughout the studies. Table 1 shows the complication rates for each of the studies.
Although fewer patients were studied following revision augmentation, long-term complication rates are significantly higher than primary augmentation. Complications such as haematoma, seroma and infection are comparable to primary silicone augmentation—overall rates are 1.5 per cent, 2.6 per cent, and 1.6 per cent respectively. Capsular contracture was higher in revision augmentation with an overall rate of 11.2 per cent (10-year rate 27.1%). Interestingly, the overall rate of rupture in revision silicone augmentation was trending slightly towards a lower rate than in primary augmentation at 3.5 per cent (10-year rate 4.1%).
The most significant measure of complications following augmentation is the re-operation rate. The overall rate of re-operation following revision augmentation was calculated to be 33.6 per cent (ten-year rate 46.7%)—significantly higher than the corresponding rate following primary augmentation (23.7%).
Cost analysis
Table 2 shows the figures for breast augmentation procedures performed in Australia per year from 2000–01 to 2014–15.2 These figures do not distinguish between primary or revision procedures, nor types of implant used. Using these figures we estimate the current rate of cosmetic breast augmentation to be 0.8 per cent of the female population.44 This equates to 1 in every 118 females in Australia.
We performed modelling to predict the complication rates occurring at one year and 10 years from the augmentations performed in 2014–15 alone. From this single year’s breast augmentations, we predict a further 2,613 re-operations would be required over the subsequent 10 years.
Figure 2 shows projected future incidence of cosmetic breast augmentation in Australia. Based on the recent trend, we have projected the rate of breast augmentation in Australian to reach over 2 per cent of the entire Australian female population by the year 2030, equating to 1 of every 38 females in Australia.
Table 3 shows the estimated cumulative complications from all augmentations performed between 2000 and 2015 and the associated cost for re-operations during this period. The number of re-operations is estimated to have been 15,251 between July 2000 and June 2015.
If cosmetic breast augmentation rates were to increase at the current rate until 2030, the cost to the Australian health system for surgical fees alone would be in excess of another A$50 million from now until 2030.
Table 4 shows the total cumulative surgical reimbursement cost directly attributable to re-operations performed from 2000-01 to 2029-2030.
Discussion
Many articles have investigated complications following the insertion of breast prostheses and compared the performance of different implant types. Most of these investigate a sole complication such as infection or capsular contracture. In 2013, an extremely thorough report by Gurgasz and colleagues45 was performed on behalf of the Australian safety and efficacy register of new interventional procedures—surgical. The results of this review support the statistical findings of our analysis. Reviews by Schaub and colleagues,46 Wong and colleagues47 and many others13,48–53 also produced results similar to ours.
Benefits
Alderman and colleagues54 describe the improvement in women’s short- and long-term satisfaction and psychosocial wellbeing following breast augmentation using the Breast-Q outcome measure. Their study concludes that breast implants are effective in improving quality of life. Their results are supported by other studies.55,56 Our study does not contradict the findings of these studies—we analysed the costs without factoring in these benefits. The literature is evolving regarding the long-term outcomes and benefits following cosmetic breast augmentation.
Cost burden
From the data we have extracted from AIHW we have demonstrated a significant financial and resource burden on the Australian health system.2 Our analysis only shows surgeons’ and surgical assistants’ Medicare rebates. We did not include complications arising from augmentations performed prior to 2000, or revision augmentations and cosmetic augmentation performed overseas. Our results are therefore a significant understatement of the real cost to the public health system and wider economy. If nothing else, this economic analysis provides a perspective regarding the use of resources and the future challenges to the Australian health sector from a growing population.
The overall re-operation rate calculated in our analysis includes 3,002 patients from a series by Araco and colleagues19 which reported disproportionately lower re-operation rates than other studies. If we removed this single article our results show even higher costs.
Livingston57 reported the cost of cosmetic tourism on a single health service over 12 months. For each patient presenting with complications from cosmetic tourism the associated cost of treatment averaged A$12,600. This study is reinforced by the findings of Miyagi58 who reported similar costs for treatment of cosmetic tourists in the United Kingdom. Adabi59 showed costs for treating complications from cosmetic tourism in the USA averaged US$18,211 per complication, equating to a cost of over US$1.3 billion on the US economy and health system.
If we attempted to calculate the total economic cost of these complications to Australia’s health system, we could apply the rates determined by Livingston and Adabi to our calculated incidence of re-operations from 2000–2015, as follows:
-
15,251 re-operations at A$12,600 (Livingston) implies a total cost of A$192 million
-
15,251 re-operations at US$18,200 (Adabi) implies a total cost of US$278 million (or A$358 million at recent exchange rates).
These figures are clearly significant. While the applicability of the results of Livingston and Adabi to Australia must be considered, these large monetary figures do illustrate the potentially greater cost of these complications than the figures we estimated. The cost of the wide range of resources involved in surgical management of complications could easily escalate the real cost to something similar to the hypothetical figures above.
For the future it is important to capture more detailed data on the cost and medical impact of cosmetic tourism, including the cost of managing complications in the public health system. This needs to be part of a wider discussion relating to the ‘movement of patients, quality assurance and standards of care, and procedures for legal responsibility’.14
We acknowledge that our study has several limitations. The first is the lack of high-quality evidence in the literature. There are no randomised trials in the meta-analysis. This paper relies heavily on retrospective and case series data. We also note that our calculations are estimates and do not represent figures from a registry or prospective dataset. Future data collected by the Australian breast device registry (ABDR) will enable more accurate modelling and calculation of the burden of disease created by cosmetic breast augmentation. The ABDR also allows tracking of device performance and complication profiles. This is of particular importance regarding conditions such as anaplastic large cell lymphoma (ALCL).
Social and psychological burden
The burden of disease created by cosmetic breast augmentation extends beyond monetary considerations; medical, social, lifestyle and psychological risks are associated with cosmetic surgery. Boulton and colleagues60 illustrated hidden risks—including medical, social and lifestyle factors—associated with cosmetic surgery, particularly in a medical system becoming increasingly market-based. A recent review by Brunton61 highlighted the negative psychological impacts of cosmetic surgery on some patients and described the various motivating factors linked to poor psychological outcomes.
Alderman62 reported a significant improvement in satisfaction with breasts, psychosocial wellbeing and sexual wellbeing in a study of 611 patients following breast augmentation. However, physical wellbeing was significantly below baseline scores both at six weeks and six months. From a prospective, multi-centre study Murphy55 reported a 95 per cent rate of satisfaction six years after augmentation and significant improvement in body image, however, he did not find any improvement in overall physical health following breast augmentation. Despite many articles describing the positive psychological impact following cosmetic breast augmentation, both Figueroa-Haas63 and Lipworth64 documented increased suicide risk and a trebled risk of death from alcoholism and the abuse of prescription and recreational drugs in women with breast augmentation.
Medical burden of breast augmentation
Complications following cosmetic breast augmentation are common. In the short-term, complications such as infection, seroma, and haematoma are expected in a low percentage of patients but become more prevalent over a longer period. Our results show that women undergoing cosmetic breast augmentation should be prepared for at least a 20 per cent rate of re-operation within ten years, with an increased risk with each year they have the implant in-situ. These results are comparable to other systematic reviews and meta-analyses.13,45–53,65,66 For patients undergoing revision augmentation, the risk of needing further surgery is even greater and expected sooner. We have found that about half of patients will require a further operation within ten years of revision augmentation.
Rarer types of complications following cosmetic breast augmentation were not taken into account such as brachial plexus impingement,67 silicone lymphadenopathy,68 chronic/late onset haematoma,69 pneumothorax and cardiac tamponade70 and recently an increased rate of ALCL detection.71,72 Many of these rare events have significant negative health effects for the patients involved and treatment commonly requires considerable time and resources.
Conclusion
This review presents complication rates following cosmetic breast augmentation with saline and silicone prostheses. While we recognise the potential benefit some women gain from cosmetic breast augmentation, we believe the medical, social, lifestyle and psychological risks are not fully appreciated in this minimally regulated market. Our analysis demonstrates that the economic costs of complications following cosmetic breast augmentation are significant, reinforcing the important role of the Australian Breast Device Registry and suggesting a need for increased regulation.73
Further investigation and more detailed economic modelling that captures the entire health care cost of complications following breast augmentation is needed. A wider discussion around the rights of patients and the responsibilities of public health systems is also required focusing on how we can modify current practices and regulations to improve patients’ awareness of the potentially significant complications as well as who should bear the financial burden. The increasing popularity of cosmetic tourism adds another significant hurdle in steps towards fuller regulation.
Acknowledgements
Vicky Tobin PhD, Monash University Plastic and Reconstructive Surgery Group (Peninsula Clinical School), Peninsula Health, Frankston, Victoria, Australia, for contribution to the study design and critical analysis.
Disclosure
The authors have no conflicts of interest to disclose.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: January 1, 2018 AEST; April 6, 2018 AEST