Introduction
Desmoid-type fibromatosis (DF), or desmoid tumour, is a rare, locally aggressive, myofibroblastic neoplasm that usually develops in musculoaponeurotic tissues and has an often-unpredictable clinical behaviour.1–3 It has a reported incidence of 2.4–4.3 cases per million and, despite being benign, local recurrence is reported in 20–65 per cent of cases at five years.2,3 Desmoid-type fibromatosis usually arises sporadically (approximately 90% of cases). While most often found in the abdominal wall, mesentery and extremities, it can occur anywhere1–6; however, DF of the chest wall and breast is exceedingly scarce.2,7 We present a case of DF found intimately adhered to and invading the vascular pedicle of a microvascular free flap breast reconstruction.
Case
Following neoadjuvant chemotherapy, an otherwise fit 56-year-old female underwent mastectomy of the left breast for HER2-positive/ER-positive, multifocal, grade 3, invasive ductal carcinoma. An immediate reconstruction was performed with a textured anatomical cohesive gel prepectoral implant. Complete pathological response to neoadjuvant therapy was noted without active malignancy identified within the breast tissue on histopathology and sentinel lymph node biopsy was negative. The postoperative recovery was complicated by breast prosthesis infection with Streptococcus pyogenes requiring removal of the prosthesis to facilitate the commencement of adjuvant radiotherapy. Eleven months after completing radiotherapy, the patient underwent a breast reconstruction with a free muscle-sparing transverse rectus abdominus myocutaneous (msTRAM) flap. The preoperative abdominal CT angiogram was unremarkable and did not identify any concerning masses. The flap artery was anastomosed end-to-end to the internal mammary artery (IMA) and two venae comitantes were anastomosed in an antegrade and retrograde fashion to the divided internal mammary vein (IMV) using two 2.5 mm venous couplers. Ischaemic time was 24 and 26 minutes without microsurgical complications. The postoperative course was unremarkable and six months later the patient underwent secondary revision with a combination of liposuction and direct excision, with improved symmetry.
At 61 years, three years later, the patient detected a new, firm, non-mobile left parasternal lump. An ultrasound identified a 27 × 19 × 33 mm mass in the msTRAM flap costal cartilage defect, a biopsy of which demonstrated a bland myofibroblastic spindle cell tumour suggestive of DF. Two weeks later, a CT angiogram was performed to define the relationship of the lesion to the free flap pedicle. The lesion extended from the sternum medially, the pleura posteriorly, intimately abutted the vascular pedicle to the msTRAM (Figure 1) and measured 32 × 22 × 43 mm. Given the increasing size and pain, the joint decision for excision was made.
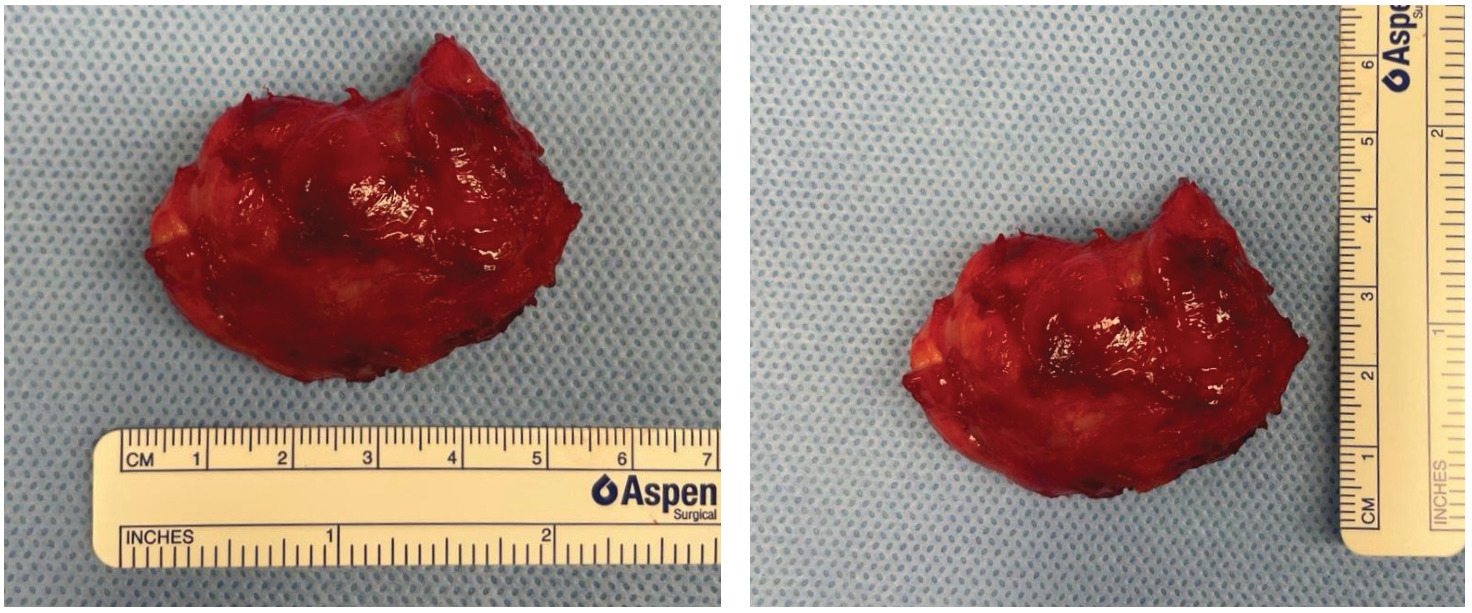
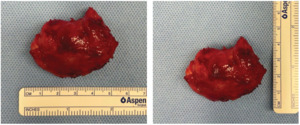
An incision was made through the superomedial inset of the msTRAM flap directly over the palpable mass, which required stripping of the sternal periosteum to free the mass (Figure 2). The pedicle to the msTRAM flap was intimately involved with the undersurface of the mass. The artery and anterograde vein were dissected free of the lesion, however, the retrograde venous coupler appeared to be surrounded by the tumour. As such, the retrograde IMV was divided and, along with the coupler, sent en bloc with the specimen for histopathology (Figure 3). The free flap was viable at the end of the surgery and there were no postoperative complications.
Histopathology demonstrated a well-circumscribed and infiltrative spindle cell proliferation composed of myofibroblasts within a loose collagenous stroma without nuclear atypia, mitoses or necrosis. The spindle cells infiltrated into the surrounding fibrofatty tissues and involved the histological margin. Immunohistochemical analysis demonstrated spindle cell nuclear straining for beta-catenin. Collectively, the above was diagnostic for DF.2 The patient was subsequently commenced on meloxicam and was changed from anastrozole to tamoxifen. The patient was closely monitored with six-monthly CT scans and showed no signs of local recurrence at one year.
Discussion
We present a DF occurring adjacent to a free flap pedicle in the irradiated chest wall of a breast cancer patient. Oh and colleagues reported a remarkably similar case: their patient had a 7 × 8 cm DF two years after delayed breast reconstruction with a free msTRAM flap following a failed tissue expander due to infection at the commencement of radiotherapy.8 In contrast, however, they elected to pursue a more radical excision, with larger surgical margins, and thus excised a larger portion of their msTRAM free flap, the entire vascular pedicle, the adjacent sternum and costochondral cartilages of ribs 2–5, achieving clear microscopic margins. Despite trialling hyperbaric oxygen, the entire remaining free flap became ischaemic, requiring debridement and secondary reconstruction with a pedicled latissimus dorsi musculocutaneous flap. On follow-up at two years, their patient remains free of recurrence, however, like ours, it is too early to suggest a cure.8
While ours is the second case in the literature of DF developing in the chest wall after an msTRAM free flap following failed implant-based reconstruction, Oh and colleagues proposed that the DF in their patient originated from the anterior rectus sheath and as such was transferred during their reconstruction. In our case, the small segment of the rectus sheath transferred with the flap was not located near the DF that developed at the site of microsurgical anastomosis. It is not possible to definitively state whether the previous failed implant-based reconstruction contributed to the development of the lesion or whether it was transferred from the anterior abdominal wall in the free flap.
With the absence of dependable predictors, the course of DF is often unpredictable.1–3 Bektas and colleagues concluded that most DF cases are characterised by an initial growth phase followed by an extended period of arrested growth. According to their estimates, 21–28 per cent of cases will spontaneously resolve (with higher rates seen in abdominal wall cases), 59 per cent will remain stable long after diagnosis and approximately 10 per cent will progress to become locally advanced where recurrence remains a significant issue, mandating operative intervention or systemic therapy.2,6 Some authors conclude that given the high local recurrence rate (up to 65%) after surgical excision, irrespective of the margin status, outcomes of surgical excision are comparable to observation alone in asymptomatic cases without risk of compromise to function.2,3,6,9,10 As a result, the traditional teachings of radical excision of DF (with some sources recommending surgical margins of greater than 5 cm) have shifted more to an initial watch and wait approach in recent years following the recommendations of the 2022 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology and the 2020 guidelines from the Desmoid Tumor Working Group (DTWG).2,3,10 Following a trial of conservative therapy, treatment is indicated in symptomatic patients with progressively enlarging masses or if there is an acute risk to adjacent structures. When surgery is indicated, while negative microscopic margins are the aim, positive margins are acceptable if necessary to maintain function, given that patients who undergo aggressive resection with widely negative margins still have recurrence rates of 16–39 per cent as the risk of recurrence is independent of margin status.3,9,10 As a result, ours appears to be the only reported case of DF in an msTRAM free flap excised without flap (and thus function) loss.
Locoregional therapy (cryoablation), antihormonal therapies (tamoxifen) and non-steroidal anti-inflammatory drugs (meloxicam) have also been used as treatments for DF, given there have been cases of spontaneous regression after their commencement,2 however, they are no longer recommended by the NCCN or the DTWG given the low-level evidence of efficacy.3,10 Systemic therapies and radiotherapy can be used for patients with symptomatic and unresectable tumours or advanced disease, however, their use depends on tumour characteristics.9,10 No international evidence-based protocols for surveillance following treatment of DF have been established.
Conclusion
Desmoid-type fibromatosis is a rare, locally aggressive tumour that develops in musculoaponeurotic tissues. While an initial watch and wait and preservation of function approach has been adopted in recent years, treatment is indicated in symptomatic patients, those with progressively enlarging masses or where there is a risk to adjacent structures. This case highlights that plastic and reconstructive surgeons should consider DF as part of the differential diagnosis when assessing patients with soft tissue lumps.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship and/or publication of this article.
_coronal__(b)_sagittal_and_(c)_axial_slices_demons.png)

_coronal__(b)_sagittal_and_(c)_axial_slices_demons.png)