Introduction
Neurotrophic keratopathy is a corneal disease characterised by reduced corneal sensation. The cornea receives sensory innervation from the ophthalmic division of the trigeminal nerve, forming the afferent limb of the corneal and lacrimal reflexes. The terminal nerve fibres secrete neurotransmitter and neurotrophic factors that promote corneal epithelial maintenance and repair.1 Neurotrophic keratopathy arises from any condition disrupting the trigeminal innervation of the cornea, resulting in progressive corneal ulceration.
Disease severity is classified according to the Mackie Classification using depth of corneal injury on slit lamp microscopy. Stage one disease is characterised by epithelial irregularity without persistent epithelial defects (punctate keratopathy). Stage two is defined by persistent epithelial defects without involvement of the deeper stroma. Stage three is characterised by ulceration into the stroma with risk of corneal melting from superimposed infection and perforation of the globe.
Traditional management ranges from topical lubrication to surgical repair of deeper ulcers but fails to address the underlying loss of protective reflexes and trophic factors. Corneal neurotisation is a surgical technique that addresses the underlying pathophysiology by the transfer of healthy donor nerve to the dennervated cornea. This article describes the first reported case of corneal neurotisation in Australia and New Zealand with objective postoperative assessment clearly demonstrating the efficacy of the technique.
Case report
An 11-year-old boy was referred with left-sided neurotrophic keratopathy from surgical debulking of a cerebellopontine angle (CPA) arachnoid cyst. Best corrected visual acuity was 6/4 in the right eye and 6/18 in the left eye. Corneal sensation measured by Cochet-Bonnet aesthesiometry was 60 mm in the right eye and 5–15 mm in the left eye. A Cochet-Bonnet aesthesiometer is a handheld device containing a retractable nylon monofilament. The pressure applied by the filament varies by adjusting its length. A reading of 60 mm implies normal corneal sensation, with shorter filament lengths correlating with reduced corneal sensation. Schirmer’s test was 25 mm for the right eye and 15 mm for the left eye. Slit lamp microscopy revealed punctate keratopathy of the left cornea, consistent with Mackie stage one disease. In-vivo confocal microscopy showed a complete absence of nerves in the left subepithelial corneal plexus. The other branches of the ipsilateral and contralateral trigeminal nerve were intact.
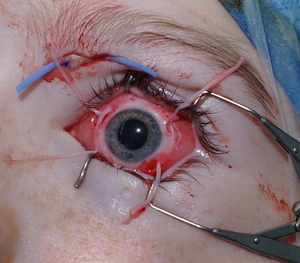
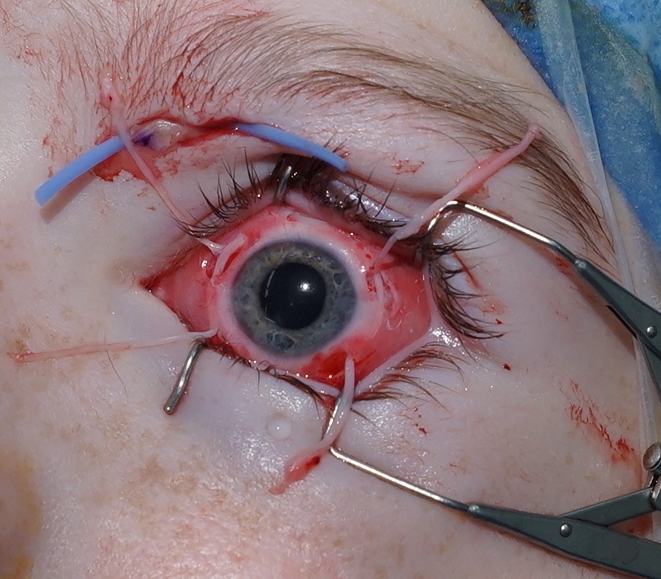
The patient and guardian consented to corneal neurotisation with a sural nerve graft under general anaesthesia. The supratrochlear nerve was exposed via a 2 cm medial infra-brow incision (Figure 1). A left split sural nerve graft was harvested taking approximately 7.5 cm in length and 50 per cent in cross-section for improved calibre matching to the supratrochlear nerve. The donor site was closed in layers with 4-0 Monocryl (Ethicon, Highway 22 N, Somerville, New Jersey, USA). The nerve graft was coaptation end-to-end with the supratrochlear nerve using 10/0 nylon. The distal sural nerve was delivered to the perilimbal space via a medial blepharotomy (Figure 1). The nerve fascicles were separated and inset into four 400-micron diameter scleral tunnels using 10-0 Vicryl sutures (Ethicon, Highway 22 N, Somerville, New Jersey, USA) (Figure 1). The conjunctival and periorbital incisions were closed with interrupted 10-0 Vicryl and 6-0 Prolene sutures (Ethicon, Highway 22 N, Somerville, New Jersey, USA), respectively. A temporary tarsorrhaphy was performed and a protective eye patch applied. The patch was removed the following day and regular chloramphenicol and dexamethasone eye drops were applied. The temporary tarsorrhaphy sutures were removed after two weeks.
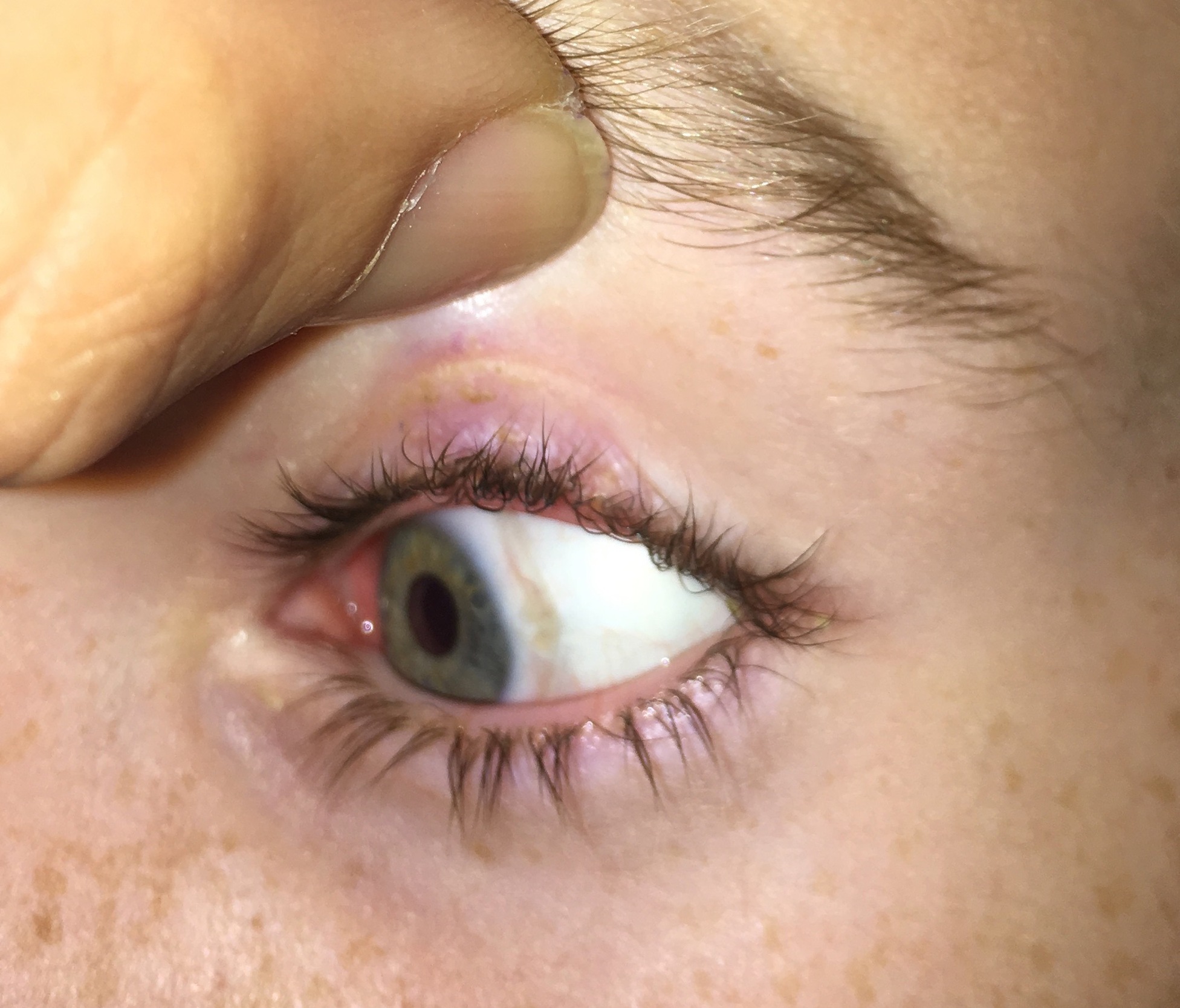
The patient was reviewed at six weeks, six months, nine months and 15 months postoperatively. The periorbital incisions healed with excellent cosmesis (Figure 2). At six weeks, there was a 2 cm strip of altered sensation on the left paramedian forehead which reduced to 1 cm at six months. The sural nerve donor site incision healed without complication. At six weeks, there was a 15 × 7 cm patch of anaesthesia along the lateral aspect of the foot and the ankle scar was mildly hypersensitive (Figure 3). At 15 months, there was a 9 × 1 cm area of reduced sensation over the lateral foot, and hypersensitivity had reduced to within 1 cm of the donor site scar. Objective improvement in corneal sensation was noted at six months and continued to improve to a final measurement of 35 mm at 15 months. Dry eye symptoms had resolved and Schirmer’s test improved to 30 mm by 12 months. Corneal punctate changes had also resolved on slit lamp microscopy by 12 months. Visual acuity in the left eye had improved to 6/12 by 15 months. Confocal microscopy at 16 months showed new nerves in the corneal subepithelial plexus.
Discussion
Corneal neurotisation was first described by Terzis and colleagues in 2009.2 They performed direct nerve transfer of the contralateral supratrochlear or supraorbital nerve through a bicoronal incision. Various minimally invasive direct nerve transfers have since been described.3 Elbaz and colleagues described minimally invasive neurotisation using a sural nerve graft to the ipsilateral or contralateral trigeminal nerve branches.4 A recent case series analysing the comparative efficacy of direct nerve transfer and nerve grafting demonstrated similar outcomes in both groups.
A nerve graft was chosen for this case to reduce the size of the periorbital skin incisions and the extent of surgical dissection in the forehead. The sural nerve is easily accessible and can be raised simultaneously to preparation of the recipient nerves. The technique is familiar to most plastic surgeons. The great auricular nerve and lateral antebrachial cutaneous nerve have been described as alternatives.5 The supratrochlear and supraorbital nerves are ideal recipients for corneal neurotisation. The location and calibre of both nerves are reliable and exceed the minimum number of myelinated axons required for nerve grafts in facial reanimation.6 The ipsilateral supratrochlear nerve was chosen for this case to minimise the regeneration distance of the donor nerve graft. An end-to-end coaptation was performed to improve the size match between the split sural nerve graft and a small-calibre supratrochlear nerve.
The efficacy of corneal neurotisation has been demonstrated in several case series. A review compiling data from 54 procedures reported a mean improvement in logMAR best corrected visual acuity of 0.41, and a mean improvement in Cochet-Bonnet aesthesiometry filament length of 38 mm.7 This is consistent with the improvement in logMAR best corrected visual acuity of 0.20, and filament length of 35 mm noted in this case. Complete resolution of Mackie stage one changes was noted on slit lamp microscopy, consistent with findings on slit lamp microscopy, specular microscopy and optical coherence tomography in previous case reports.8 Regeneration of the subepithelial nerve plexus was demonstrated on in-vivo confocal microscopy. Ex-vivo histopathological assessment of neurotised corneas and functional neuroradiological imaging have shown that these new nerves have normal ultrastructure and function.9,10
Children show significantly greater improvement in corneal sensation and visual acuity than adults, probably reflective of greater capacity for nerve regeneration, central nervous system plasticity and absence of confounding medical comorbidities. Corneal neurotisation has been used in adults in many cases series with excellent clinical results but is unlikely to be beneficial for patients with impaired capacity for nerve regeneration.
Conclusion
Corneal neurotisation is a new and promising treatment for a neurotrophic keratopathy. This is the first reported case of corneal neurotisation in Australia and New Zealand. The case clearly demonstrates the feasibility of the technique, donor site morbidity and expected timeline for improvement in corneal epithelial sensation, innervation and repair.
Patient consent
Patients signed informed consent regarding publishing their data and photographs.
Conflict of interest
The authors have no conflicts of interest to disclose.
Financial declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: 2020 August 13