Introduction
Facial paralysis is a disfiguring condition that significantly impacts on a patient’s quality of life. The ultimate goal of facial reanimation is for patients to achieve symmetry, synchronicity and spontaneity in facial movements.1–9 Traditionally, dynamic facial reanimation procedures have been reserved for younger patients. Static reconstructions are more commonly performed in elderly patients, based on the belief that they will have poorer functional outcomes following complex dynamic reanimation procedures.3 Nevertheless, with ageing patient populations and increasing life expectancies, we believe there is a greater role for dynamic facial nerve reconstruction in the elderly.
Within the literature, hypoglossal nerve transfer with nerve grafts are the most commonly described method of dynamic reanimation.3,9 However, its disadvantages include potential hemiglossal atrophy and impairments to speech and oral intake, as well as the need to harvest a nerve graft.8 In contrast, masseteric-to-facial nerve transfer can be performed with direct coaptation of the descending part of the nerve to masseter (from the trigeminal nerve) to branches of the facial nerve. While first described by Spira in 1978,7 masseteric-to-facial (MTF) nerve transfer has only recently gained popularity.2
The advantages of MTF nerve transfer for midface and perioral reanimation are well described.10 While initially not spontaneous, this technique has the ability to rapidly produce a smile, that, with practice has the potential to become more spontaneous and natural. This is owing to the high density of motor axons in the nerve to masseter (producing a powerful muscle contraction), the ability to perform primary nerve coaptation in close proximity to target muscles, as well as communications between the facial and trigeminal cortical centres which could potentially facilitate cerebral adaptation.2–5,7,9,10 Preoperative electromyography can be used to detect involuntary activation of the masseter during spontaneous or voluntary smiling.11,12 The presence of which has been shown to predict the success of MTF transfer in producing a spontaneous smile.11,12 There is also minimal donor site morbidity as the synergistic masticatory functions of the temporalis are maintained and the proximal motor branches of the nerve to masseter are preserved thus preventing complete denervation of the masseter.5,9,10
Despite its many benefits, MTF nerve transfers in individuals aged greater than 60 years are not widely reported in the literature. This present study aims to evaluate the safety and outcomes of masseteric-to-facial nerve transfer in the elderly population.
Materials and methods
Literature review
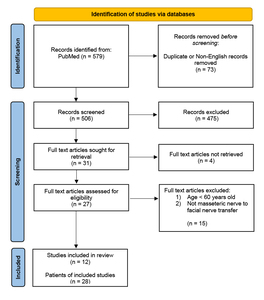
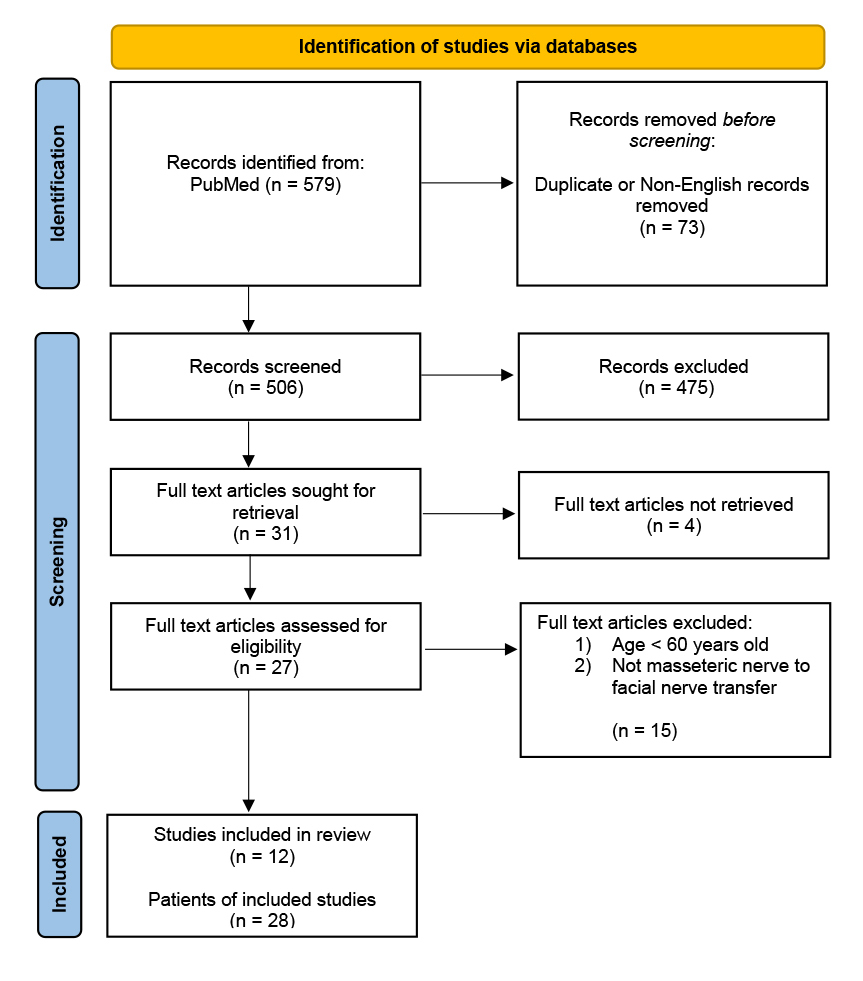
A literature search was performed using the PubMed database for articles within the inclusion period of 1 January 1978 to 31 December 2018. The defined terms used included: ‘masseteric nerve’; ‘nerve to masseter’; ‘masseter nerve’; ‘trigeminal nerve’; ‘reanimation’ and ‘facial palsy’; ‘facial paralysis’. Results were confined to literature in the English language. Articles were first screened by their title and abstracts. This was followed by closer evaluation of the article to include only those that described strict nerve to masseter to facial nerve transfers and had patients aged 60 years or older in their series. The selected articles were reviewed for the patient demographics, methods of reconstruction, cause of paralysis, duration of paralysis and comments on complications and outcomes. Articles were excluded if patient demographics, surgical details and outcomes were not available.
Clinical series
A retrospective chart review of all patients who underwent a MTF nerve transfer from January 2011 to December 2018 from a single centre was conducted. An ‘elderly patient’ was defined as an individual aged 60 years or greater. All clinical notes were evaluated for patient demographics, cause of facial nerve palsy, extent of palsy, duration of palsy, associated procedures, outcomes and complications. All data collected was de-identified and analysed using Microsoft® Excel software version 15.31 (Microsoft Corporation, Redmond, Washington, USA). Ethics approval for the study was obtained through the Monash Health Human Research Ethics Committee [RES-18-0000-768Q].
Results
Literature review
Of the 506 articles identified, only 12 met the inclusion and exclusion criteria. From these studies, a total of 29 patients who were aged 60 years or older and underwent a MTF nerve transfer were identified. One patient was excluded due to lack of data within the manuscript, leaving 28 patients for review.
There were 12 men and 16 women with a mean (SD) age of 68 (8.3) years old (Table 1). Patients were followed up postoperatively for a mean (SD) of 18 (13.4) months. The majority of nerve transfers (26/28, 93%) were delayed reconstructions. The mean (SD) duration of facial nerve paralysis prior to nerve transfer was 17 (37, range 0–204) months. The most common cause of facial paralysis reported was an acoustic neuroma, reported in 12 of 28 (43%). In the majority of cases, the masseteric nerve was coapted to distal branches of the facial nerve. In 14 (50%) patients, the masseteric nerve was coapted to both buccal and zygomatic branches, in six (21%) patients to buccal branches only, in one (4%) patient to zygomatic only, in six (21%) patients to the buccal branch via nerve graft, and in one (4%) patient to the lower trunk of the facial nerve.
Five (18%) patients also underwent other concurrent facial nerve palsy procedures. Three patients had a concurrent gold weight inserted into their ipsilateral upper eyelid; one had an oral commissure suspension by de-epithelialising the skin; and in Spira,7 one patient underwent a temporalis transfer for lagophthalmos and fascia lata sling.
Postoperative outcomes were variably reported. The time to first facial movement from surgery was described in 15 patients with an average (SD) of 5.5 (2.3) months. Improvement in oral commissure excursion was only available in seven cases, with an average (SD) increase in excursion of 11 (5.7) mm. The Terzis scoring system was used in one case series,3 while the Sunnybrook grading system was used in another.13 Both case series demonstrated overall improvements in facial symmetry post MTF nerve transfer. The remaining case studies used qualitative descriptions in postoperative function examining facial symmetry, nasolabial fold symmetry and spontaneity of smile. When reported, follow-up periods ranged from three to 68 months (average 17.5 months), with additional revision procedures noted in several studies.3,7,14 No complications were reported in the 28 patients.
Clinical series
During the study period, 16 patients underwent a MTF nerve transfer at our institution. Of these, 11 patients met the age inclusion criteria and were included in the clinical series. There were 10 males and one female with a mean (SD) age of 78 (7.4) years old (Table 2). In the majority of cases (10/11, 91%), the cause of facial nerve paralysis was resection of a parotid malignancy with facial nerve sacrifice, and the masseteric nerve transfer was completed immediately in the same operation. In contrast to the literature review, the vast majority of nerve transfers were immediate reconstructions in the clinical series. The remaining one masseteric-to-facial nerve transfer was performed for an acoustic neuroma and the duration of the facial nerve palsy was six months.
In seven (64%) patients, the nerve to masseter was coapted with distal buccal branches of the facial nerve, in two (18%) patients it was coapted with buccal and zygomatic branches, and in a further two (18%) patients, the nerve to masseter was coapted to the buccal and marginal mandibular branches via a sural nerve graft. In all 11 cases, MTF nerve transfer was performed concurrently with other facial reanimation procedures. Eight (73%) patients had fascia lata slings inserted concurrently to suspend the ipsilateral oral commissure. As a nerve to masseter transfer to the facial nerve does not provide background muscle tone, fascial slings were concurrently used to help create symmetry in repose.
The average duration of the head and neck resection including the nerve to masseter transfer was 8.5 hours, with an average (SD) hospital length of stay of 10 (7.2) days. This was due to the fact that the majority of nerve transfers occurred in the setting of major head and neck resections and reconstructions. Of the 11 patients, three (27%) suffered a postoperative complication: one had pneumonia, one had recurrent seromas and one patient had postoperative delirium secondary to atelectasis. There were no mortalities in this series.
Patients were followed up postoperatively for an average (SD) of 18 (17.1) months. Unfortunately, no consistent measure was used to assess pre- and postoperative facial nerve function.
Discussion
There is a growing body of evidence, mostly in the form of clinical case series, describing the benefits of MTF nerve transfer for facial reanimation following facial nerve paralysis.1,4–6,9,10,14,16–18,20–25 Our retrospective clinical series is the largest to date, contributing a further 11 patients to the existing 29 cases and further supporting the positive findings of previous published studies.3,6,7,9,10,14,15,18,20,22,25,26 Nevertheless, there is still a gap in the literature when it comes to its use in elderly populations. This is likely due to the misconception that dynamic reconstructions in elderly patients yield suboptimal results, and that similar functional outcomes can be achieved with shorter, less morbid, static procedures.3,27 There is also a hesitation in offering complex procedures to elderly individuals due to a fear of increased complications and a perceived inability of older patients to tolerate longer operations.3,27
However, dynamic facial reanimation is superior to static facial nerve reconstruction as it corrects asymmetries of the face both at rest and also during facial expression, allowing for a more natural appearance.1 Static reconstructions also have the disadvantage of being affected by progressive laxity, often requiring revision.3 In addition, MTF nerve transfer is a relatively quick, dynamic reanimation procedure. Rubi and colleagues have even described the procedure being performed as a 70 minute day case under local anaesthetic with intravenous sedation.13
Indeed, concerns regarding the safety of this procedure in the elderly can be quelled by examining its complication profile. Of the 28 cases of MTF nerve transfer included in the literature review, there were no complications and minimal donor site morbidity. In the clinical series, we reported a 27 per cent complication rate, two systemic and one surgical site, though these occurred in the setting of major head and neck malignancy resections and reconstructions, with an average total operative time of 8.5 hours. Thus, the disparity in complications between the two arms of the study can be attributed to the fact that the nerve transfers in the literature review were performed as isolated procedures separate to the oncological resection.
One of the arguments against dynamic facial reanimation in the elderly is based on evidence demonstrating poorer nerve regeneration in the elderly.9,26 From our literature review, the average time to first facial motion following MTF nerve was 5.5 months in patients greater than 60 years, compared to the 3.8 months in the general population.27 Postoperative commissure excursion in the elderly averaged an 11 mm improvement, compared with the reported 7–12 mm improvement among younger patients. While these findings demonstrate that nerve recovery is slower in older patients, the overall smile scores achieved between young and old patients are similar,3,9,27 suggesting that MTF nerve transfer in the elderly is still a valuable procedure.
Current guidelines recommend that nerve transfers should be performed within 12 months of facial paralysis onset for best functional outcomes.3 In our clinical series, 10/11 (91%) of the patients underwent immediate MTF nerve transfer at the time of the resection of their parotid malignancy. We believe that the MTF nerve transfer is an ideal reanimation procedure in the setting of parotidectomy with facial nerve sacrifice, as much of the dissection has already been performed.
Limitations
Several caveats exist in the interpretation of the results in this study. Within the literature review, there was significant variability in the measured outcomes and inconsistency in the standardised facial nerve grading scores used. These differences in the quantitative outcomes measured between the studies made it difficult to accurately compare the reviewed studies.
Similarly, the literature review and clinical series cohorts had different durations of facial nerve palsy and are not directly comparable. In the clinical series, the majority of the nerve transfers occurred as an immediate procedure post parotidectomy, while in the literature review, the majority of cases were delayed procedures for facial nerve palsy secondary to an acoustic neuroma. In addition, there was no standardised means to assess the effectiveness of the MTF nerve transfer in the clinical series.
It can be seen that the current evidence for MTF nerve transfer in the elderly is promising but remains limited. Our study was able to contribute the largest clinical case series of patients aged greater than 60 years, and supports the use of this procedure for dynamic facial reanimation. Looking to the future, larger, prospective studies with stringent examination of functional outcomes and complications are required. Additionally, evaluation of patient reported outcomes will add greater evidence to the true value of a procedure which seeks to improve function and quality of life for patients.
Conclusions
Despite its many benefits, MTF nerve transfers in individuals aged greater than 60 years are not widely reported in the literature. The present study adds to the growing body of evidence that MTF nerve transfer is a safe and effective procedure for dynamic smile restoration in elderly patients and should be considered for all patients with short-term facial nerve palsies.
We believe that MTF nerve transfer is a simple and effective technique in restoring dynamic smile function, improving form, function and quality of life in elderly patient populations.
Conflict of interest
The authors have no conflicts of interest to disclose.
Financial declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: 2020 April 30; 2021 March 02