Background
Salivary tumours are uncommon, comprising only 2–5 per cent of head and neck neoplasms,1 with mucoepidermoid carcinoma (MEC) being the most common salivary cancer in both adults and children.1–4 Clinically, head and neck MEC can present variably from being asymptomatic to locally or metastatically aggressive.5,6 Treatment is primary surgical resection with neck dissection. The use of adjuvant radiotherapy is indicated for patients at high risk of recurrence, such as those with a high tumour stage, positive resection margins and high histological grading.6–8
Case report
A 57-year-old otherwise well male presented with a six-month history of a progressive, non-tender right parotid swelling associated with occasional right-sided tinnitus and headaches. There was normal cranial nerve function and no cervical lymphadenopathy. Fine needle aspiration cytology demonstrated MEC. Magnetic resonance imaging and CT scans showed a 1.5 cm tumour within the superficial lobe of the right parotid gland and a bulky tumour within the markedly distended retromandibular vein (RMV) and its tributaries within the masseter muscle and those that extend medially around the vertical ramus of the mandible into the infratemporal fossa towards the pterygoid plexus. The tumour within the distended RMV and common facial vein (CFV) extended into the internal jugular vein (IJV) from the level of the angle of the mandible to the level of the cricoid cartilage (Figure 1a and b). The RMV was not connected with the external jugular vein, which was free of tumour. Staging CT showed no nodal metastasis or disease elsewhere.
The patient was managed by a head and neck multidisciplinary team and underwent a right total parotidectomy with facial nerve preservation, resection of the masseteric muscle and the infratemporal fossa and ipsilateral modified radical neck dissection, achieving clear macroscopic margins. The IJV was ligated superiorly below the skull base and inferiorly below the level of the hyoid bone and was removed en bloc with the parotidectomy and neck dissection specimen (Figure 2a–d). The tumour appeared to be entirely within the aforementioned greatly distended venous network as one contiguous metastatic deposit without obvious involvement of the parotid gland.
Histology demonstrated an intermediate-grade MEC within the superficial lobe of the parotid gland (Figure 3a and b) with sheets of necrosis present within the tumour. The tumour cells were predominantly mildly to moderately pleomorphic, with occasional markedly pleomorphic cells. Periodic acid-Schiff-diastase staining showed intracytoplasmic mucin. There was extensive intravenous tumour with no direct extension from the primary tumour into the adjacent blood or lymphatic vessels and there was no nodal metastasis. Tumour was present at a resection margin microscopically within a single vein deep to the masseter muscle.
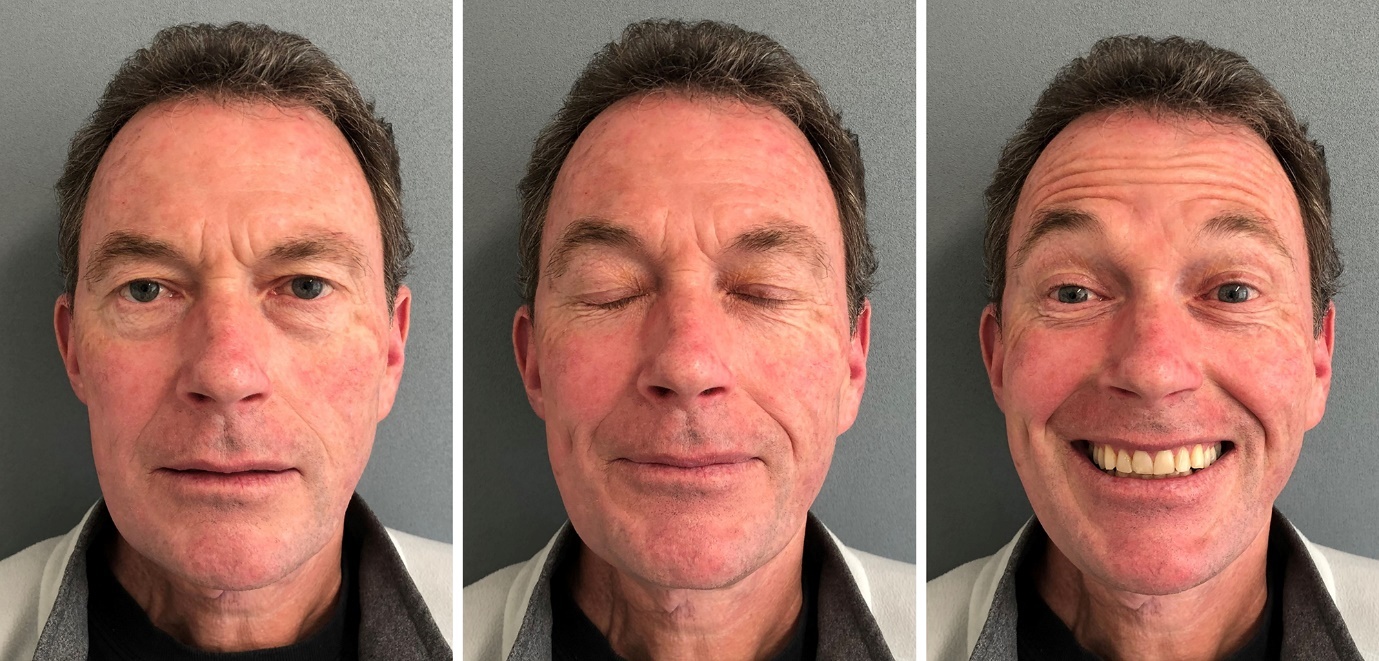
The patient was followed up by the multidisciplinary team. Apart from temporary partial neuropraxia of the right facial nerve, the postoperative course was uneventful and the patient was discharged on day four and subsequently completed adjuvant radiotherapy as part of the original treatment plan. At follow-up 14 months postoperatively, the patient had normal facial nerve function and no clinical sign of local or regional recurrence (Figure 4).
Discussion
Risk factors for developing MEC include previous radiation exposure, smoking and viral infections such as human papillomavirus.2,9 Histological grading provides the best prognostic factor for survival, with high-grade tumours being the most aggressive.9,10
Mucoepidermoid carcinoma tends to disseminate initially via lymphatic channels which are typically thin-walled and leaky compared with the multi-layered blood vessel walls.1,5 Similarly, cancer cells that spread haematogenously enter the small capillaries then larger vessels via the venous network. The propensity for lymphatic or haematogenous invasion with metastatic MEC has not been fully identified but is thought to be related to tumour cell markers and enzymes.4,5
Tumours that commonly metastasise intravascularly include renal cell carcinoma and hepatocellular carcinoma.5 Histological evaluation may identify penetration of small vessels adjacent to the primary tumour, which suggests the development of metastatic disease.5 In the case presented, the behaviour of the tumour was unusual—a small primary tumour with extensive intravenous involvement without direct invasion of the veins, perivascular or lymphatics. Although it is possible that lymphatics could have carried the tumour cells into the neck veins via small connecting channels, neither lymphatic nor capillary involvement was observed.
Conclusion
This case of MEC of the parotid is unusual in that a small primary tumour was associated with extensive ipsilateral intravenous metastasis without direct invasion of the involved veins.
Acknowledgements
The authors would like to thank Dr Claire French, pathology registrar at Wellington SCL, Hutt Hospital, New Zealand, Dr Hannah Kim, radiologist and Dr Eric Ji, radiation oncologist, Wellington Regional Hospital, New Zealand, for their assistance.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: November 7, 2020 AEST
_and_more_anteriorly_(b)__demonstratin.jpeg)
_surgical_markings_with_shaded_area_indicating_the_palpable_tumour_.jpeg)
_the_primary_tumour_within_the_parotid_gland_showing_mi.jpeg)

_and_more_anteriorly_(b)__demonstratin.jpeg)
_surgical_markings_with_shaded_area_indicating_the_palpable_tumour_.jpeg)
_the_primary_tumour_within_the_parotid_gland_showing_mi.jpeg)