Introduction
Posterior upper quadrant trunk defects are challenging to reconstruct. The LD myocutaneous flap is the most common reconstructive option used. It offers a reliable flap with a large skin paddle and good arc of rotation. It does, however, have the significant donor site morbidity of a muscle harvest and propensity for seroma formation. This case series demonstrates the versatility of the parascapular flap in reconstructing a range of defects overlying the paraspinal region, scapula, deltoid and axilla. The size of the defect, relationship to the pivot point and laxity of the donor site were the main determinants of the suitability of this flap as a reconstructive option. There is a learning curve to this procedure, but the advantages include muscle-sparing, rapid recovery and minimal seroma formation.
Case series
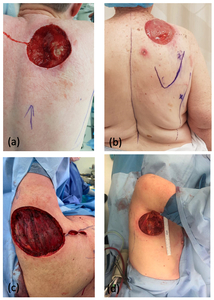
We describe six cases of defects of the posterior upper quadrant which were reconstructed with a parascapular flap at a single institution between 2018 and 2020. Patients were followed up at three weeks, six weeks and three months postoperatively.
Preoperatively, the midline, scapula outline and lateral edge of the LD were marked with Doppler identification of the circumflex scapular artery. The circumflex scapular vessels were located lateral to the scapula as it exits the triangular space, two-fifths along a line drawn from the midpoint of the spine of the scapula to the scapula tip.1 Patients were positioned in the lateral position with the arm placed in a thoracic arm rest.
The anterior margin of the flap was raised to expose the circumflex scapular vessels within the triangular space, or the advancing edge of the defect was used as an initial exploratory window. Once identified, the flap was raised in a subfascial plane from distal to proximal. The transverse branch of the circumflex scapular vessels, smaller branches to teres major and minor and scapula periosteal branches were ligated to gain pedicle length. Division of these branches was key to minimise kinking of venous outflow at transposition of the flap into the defect. The flap was rotated in the direction which created a smaller angle between the axis of the flap donor and recipient sites. The donor site was closed primarily if possible and drains were placed into the donor and recipient sites. Patients were immobilised for three days prior to commencing active range of motion.
Table 1 highlights patient and operative details for the six cases. The average patient age was 70 years. The defects ranged in size from 8 × 8 cm up to 10 × 15 cm. The defects were located in the axilla (Figure 1a), in the deltoid region (Figure 1b), over the scapula (Figure 1c) and in the superior paraspinal region (Figure 1d). The superior margin of the scapula defects extended to the superior edge of the trapezius. Flaps ranged from 6–10 cm in width to 15–26 cm in length, and all donor sites were closed primarily over drains.
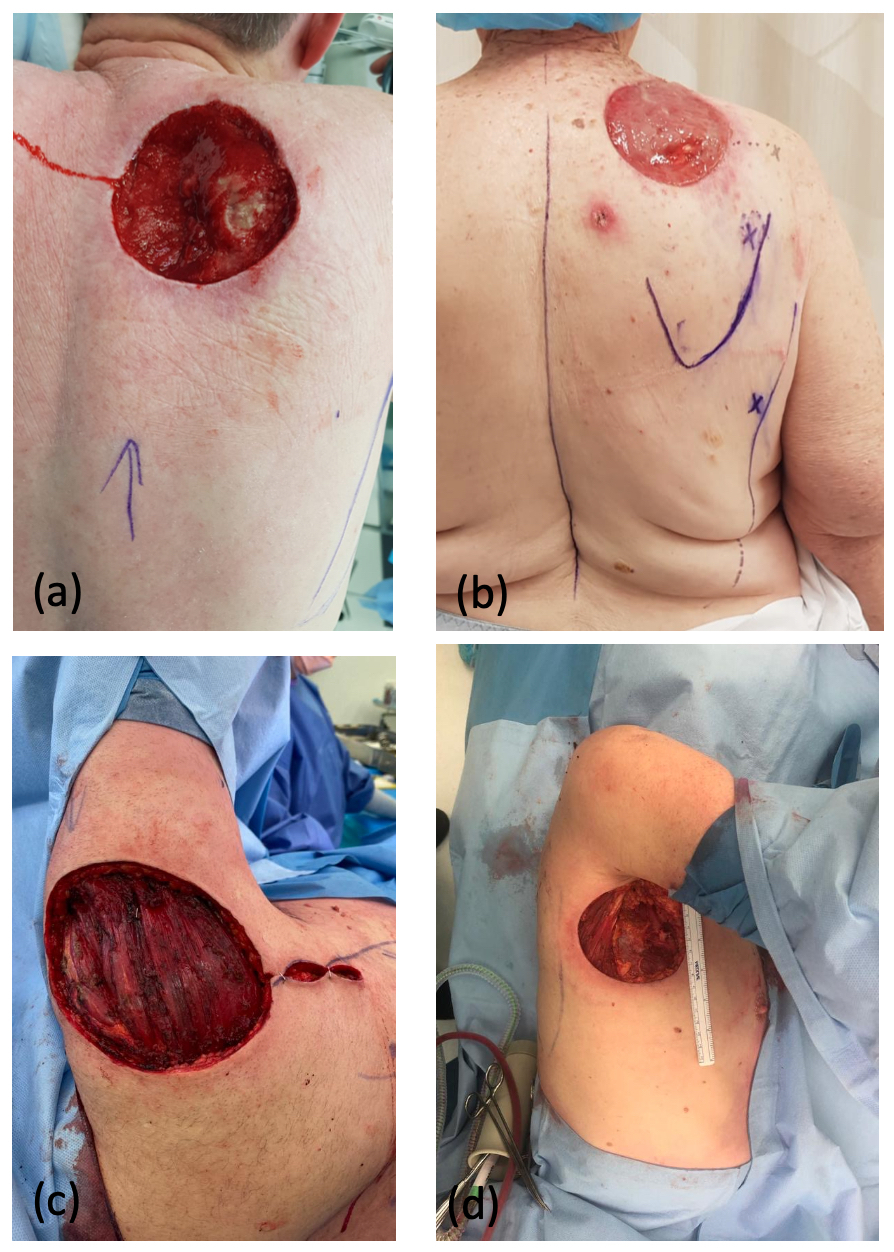
Case two was particularly interesting as the patient had an area of atrophy and non-blanching macular pigmentation over his right LD region, which was the planned donor site (Figure 2a). He reported that the skin changes were present over a number of years and recalled no trauma to the site. He had reduced bulk within his LD muscle, albeit full power (Medical Research Council [MRC] Grade 5). Preoperative CTA (Figure 2b) and an ultrasound scan demonstrated atrophy of both the LD and overlying subcutaneous fat, with no evidence of a vascular malformation. The subscapular axis was intact, but perforators through the LD were not visualised or isolated with a Doppler probe. Intraoperatively, thoracodorsal artery perforators were absent. The circumflex scapular pedicle was of good calibre and the decision was made to proceed with a parascapular flap, which healed without complication.
The average length of hospital stay was five days (range three–11 days). Case three was excluded from this as the patient developed flap congestion postoperatively and returned to theatre on the day of surgery. The flap was returned to its original location and delayed. Two weeks later the flap was raised and the pedicle was dissected further to include division of the transverse branch of the circumflex scapula. Although the donor site was amenable to primary closure, it placed some pressure on the pedicle and a small split skin graft was preferred. All other cases recorded no complications or seroma. Range of shoulder movement returned to baseline within six weeks postoperatively.
Discussion
Regional flaps remain a mainstay for reconstruction of posterior trunk defects due to the paucity of recipient vessels for free flap reconstruction. The upper thoracic region encompassing the scapula and intervening area of the back is thought to be best served by the LD muscle or myocutaneous flap, supplied by the thoracodorsal pedicle.1,2 Circumflex scapular or thoracodorsal artery perforator (TDAP) flaps are recognised as a secondary, ‘back-up’ option.
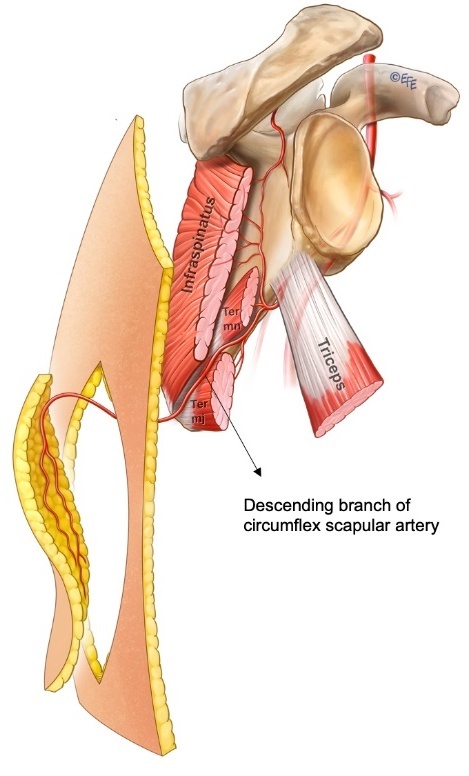
Fonseca dos Santos first described the anatomy of an axial pattern flap based on a cutaneous branch of the circumflex scapular artery in 1980.3 The circumflex scapular artery originates from the subscapular artery with the cutaneous branch passing through the triangular space, which is bounded by the teres minor superiorly, the long head of the triceps laterally and the teres major inferiorly (Figure 3). dos Santos suggested that the flap should be based transversely, but Gilbert recommended an oblique flap.3 Further anatomical work by Ohsaki and Maruyama in 1992 demonstrated the consistent ascending, horizontal and descending cutaneous branches of the circumflex scapular artery and illustrated the communication between the descending branch with musculocutaneous perforators of the intercostal and thoracodorsal arteries.4 With the capture of this descending cutaneous branch and adjacent angiosomes, parascapular flaps of up to 30 cm in length can be raised.5,6 In this case series, the longest parascapular flap was 26 cm with no flap necrosis.
We aim to highlight the versatility of pedicled parascapular flaps in reconstructing various large soft-tissue defects around the axis of the scapula, with primary closure of the donor site. It is our preferred choice for defects in the posterior upper thoracic region, replacing like with like by using excess tissue overlying the lower back and sparing the LD muscle. We have demonstrated that parascapular flaps can be safely and reliably used for defects around the axilla and deltoid, over the scapula and even up to the midline (Figure 4). No intraoperative position change is necessary and the duration of surgery is relatively short. The donor site is closed primarily and minimal muscle dissection renders a quicker overall recovery with minimal functional compromise as supported by other studies,7 which is particularly useful for older patients as in this case series. Minimal dead space is created by avoiding the LD harvest and we propose that this minimises seroma formation.
An important observation when using this flap for regional reconstruction is that the width of the flap needs to almost match the width of the defect. In this case series, the flap widths were at most two centimetres smaller than the narrowest dimension of the flap. The flap does not stretch, and often the defect has minimal laxity. We recommend as close to a 1:1 flap width to defect ratio as possible, which serves as a planning guide when contemplating donor site primary closure. A second important point is the complete dissection of the pedicle including ligation of the muscle, periosteal and transverse cutaneous branches of the circumflex scapular vessels to avoid pedicle kinking and flap congestion. This proved to be a learning curve as all further cases were complication and congestion free.
If the defect precludes the use of a parascapular flap, a TDAP could be used to reconstruct similar defects. In another case, an 81-year-old had a defect overlying the scapula measuring 10 × 15 cm. The defect involved the circumflex scapular axis, preventing its use. A TDAP flap measuring 10 × 25 cm was safely transposed into the defect with primary closure of the donor site. If both parascapular and TDAP flaps are available reconstructive options, our preference would still be the parascapular flap as it has a more consistent and predictable blood supply, and leaves the LD as a backup option based on the thoracodorsal artery.
Conclusion
As perforator flaps continue to gain popularity, we argue that vertical subscapular perforator flaps—that is, parascapular or TDAP flaps—are ideal options for upper thoracic defects. We have demonstrated that these flaps are versatile and can extend out to the axilla, deltoid and paraspinous regions. Although these flaps are more technically challenging than a standard LD, the muscle-sparing nature limits donor site morbidity and we regard them as workhorse flaps, minimising the reliance on the LD flap.
Acknowledgements
Dr Levent Efe, certified medical illustrator, for the illustrations.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: October 18, 2020 AEST
_image_of_case_two_demonstrating_skin_changes_and_atrophy_over_the_planned_donor_site_(rig.jpeg)

_p.jpeg)
_image_of_case_two_demonstrating_skin_changes_and_atrophy_over_the_planned_donor_site_(rig.jpeg)
_p.jpeg)