Introduction
Infections are a common and serious complication following burn injury.1,2 Burn wounds provide an ideal medium for bacterial proliferation and are a direct portal of entry through the skin barrier. Hypermetabolic states and immunosuppression further predispose burns patients to developing infection. Burn wound infections can delay healing, increase scarring and lead to systemic infection, with sequelae of multi-organ failure and death.1
Perioperative antibiotic prophylaxis is defined as the use of antibiotics during the preoperative, intraoperative and postoperative periods to reduce intraoperative bacteraemia and prevent surgical site infections. Evidence for its use in burns surgery is limited.3–5 Early studies into antibiotic prophylaxis for burns surgery were performed in the setting of delayed wound debridement, an outdated practice.6–8 In current practice, the majority of acute burns are clean due to mechanism of injury or use of preoperative antimicrobial dressings.9 There has also been a shift towards early debridement and wound closure, reducing the window for secondary colonisation and wound infections.10 This practice has resulted in a reduction in rates of wound infection and sepsis and called into question the indication for antibiotic prophylaxis.1,10
Excessive use of antibiotics, particularly as prophylaxis for surgery, has become a growing concern.11 It can contribute to the development of antibiotic resistance, clostridium difficile infections and adverse reactions.12,13 For burns patients, who often require multiple surgical procedures to remove devitalised tissue and prepare the wound bed for grafting, antibiotic resistance can impede wound healing, graft take and prolong hospital admissions.5 It can also result in the development of complications such as sepsis and multiple organ dysfunction.14 Resistant organisms have now emerged as the leading cause of death from infection in burns patients.2 Furthermore, antibiotic resistance is a developing major public health concern.
There are currently no consensus guidelines surrounding the appropriate use of perioperative antibiotic prophylaxis in acute burns surgery. Current Australian therapeutic guidelines advocate for single dose prophylaxis for the majority of clean and clean-contaminated procedures, but acknowledge that if postoperative doses are still required, despite lack of quality evidence, then prophylaxis should continue for 24 hours.15 A recent Australian study found high rates of inappropriate perioperative prophylactic antibiotic use.11 Our unit’s policy is to use cephazolin for acute burns or if there is no growth on wound culture, or piperacillin and tazobactam for contaminated wounds at induction. Further perioperative antibiotic use is dependent on a combination of wound culture results and the clinical scenario. The primary aim of this study was to determine current prescribing practices for perioperative antibiotic prophylaxis at the Victorian adult burns service (VABS) and the factors that influenced these decisions. The secondary aim was to observe whether the duration of antibiotic prophylaxis influenced the risk of postoperative wound infection and/or bacteraemia. The results of this study may help to guide future trials and develop guidelines for appropriate perioperative antibiotic prophylaxis in burns patients.
Methods
Setting
The VABS at the Alfred Hospital is a state-wide provider of burns care for adults with complex or major burns, serving a population of 5.5 million in Melbourne, Australia.
Study design
We conducted a retrospective chart review of perioperative antibiotic prophylaxis practices at the VABS from November 2018 to November 2019.
Inclusion criteria
We included all adult acute burns patients admitted under the VABS who had an operation and collected data from the perioperative period surrounding the first operation. This period included the preoperative phase from admission to the first operation, the intraoperative phase and postoperative phase extending up to two weeks post operation. Any patients who died or were palliated within the first 48 hours were excluded.
Data collection
Basic demographic data, burn specific data and perioperative antibiotic use data were collected. The outcome measures were wound infection, bacteraemia, other infections and the presence of resistant organisms. Data was extracted from the VABS database and manually through a retrospective chart review from the hospital’s electronic medical records by an individual data collector.
Definitions and study outcomes
Perioperative antibiotic prophylaxis was defined in our study as antibiotics commenced at the time of surgery and continued up to the first graft or wound check. At our unit this check is typically done no later than five days postoperatively. Further antibiotic use beyond this point was considered as treatment rather than prophylaxis for the purpose of this study. Wound infection was defined as colonisation with a positive wound swab from the operative site within two weeks of the operation and the use of antibiotics to treat this. Use of antibiotics for treatment of a wound infection included continuation of antibiotics beyond the initial perioperative prophylaxis course of up to five days, recommencement of antibiotics and commencement of a new antibiotic. Antibiotic use as prophylaxis for subsequent operations was excluded from this. Bacteraemia was defined as the presence of positive blood cultures postoperatively within two weeks of the operation and commencement of antibiotics for this. Other infections were identified through positive growth from other sites such as sputum and urine and the commencement of antibiotics for this. Presence of resistant organisms was defined as the presence of methicillin resistant Staphylococcus aureus (MRSA), vancomycin resistant Enterococcus (VRE), carbapenemese producing Enterobacteriacae (CPE) or other resistant organisms acquired during acute admission or identified within three months of discharge.
Statistical analysis
Continuous variables were summarised using means and standard deviations or medians and interquartile ranges depending on the underlying distribution of the data. Categorical variables were reported as counts and percentages. Univariate analyses were conducted using Student’s t-test for normally distributed continuous variables, Wilcoxon rank sum test for non-normally distributed continuous variables and Chi-square or Fisher’s exact test as appropriate for categorical variables.
Results
During the study period, 222 patients were admitted to the VABS requiring operative management for an acute burn injury.
Perioperative antibiotics was given to 219 patients: 190 patients received cephazolin or cephalexin, 15 patients received piperacillin and tazobactam, 14 patients received other antibiotics, three patients did not receive any perioperative prophylaxis (Table 1).
Patients who received piperacillin and tazobactam were older in age than those who received cephazolin or cephalexin (58.3 ± 19.6 versus 45.3 ± 17.8, p = 0.02) (Table 2).
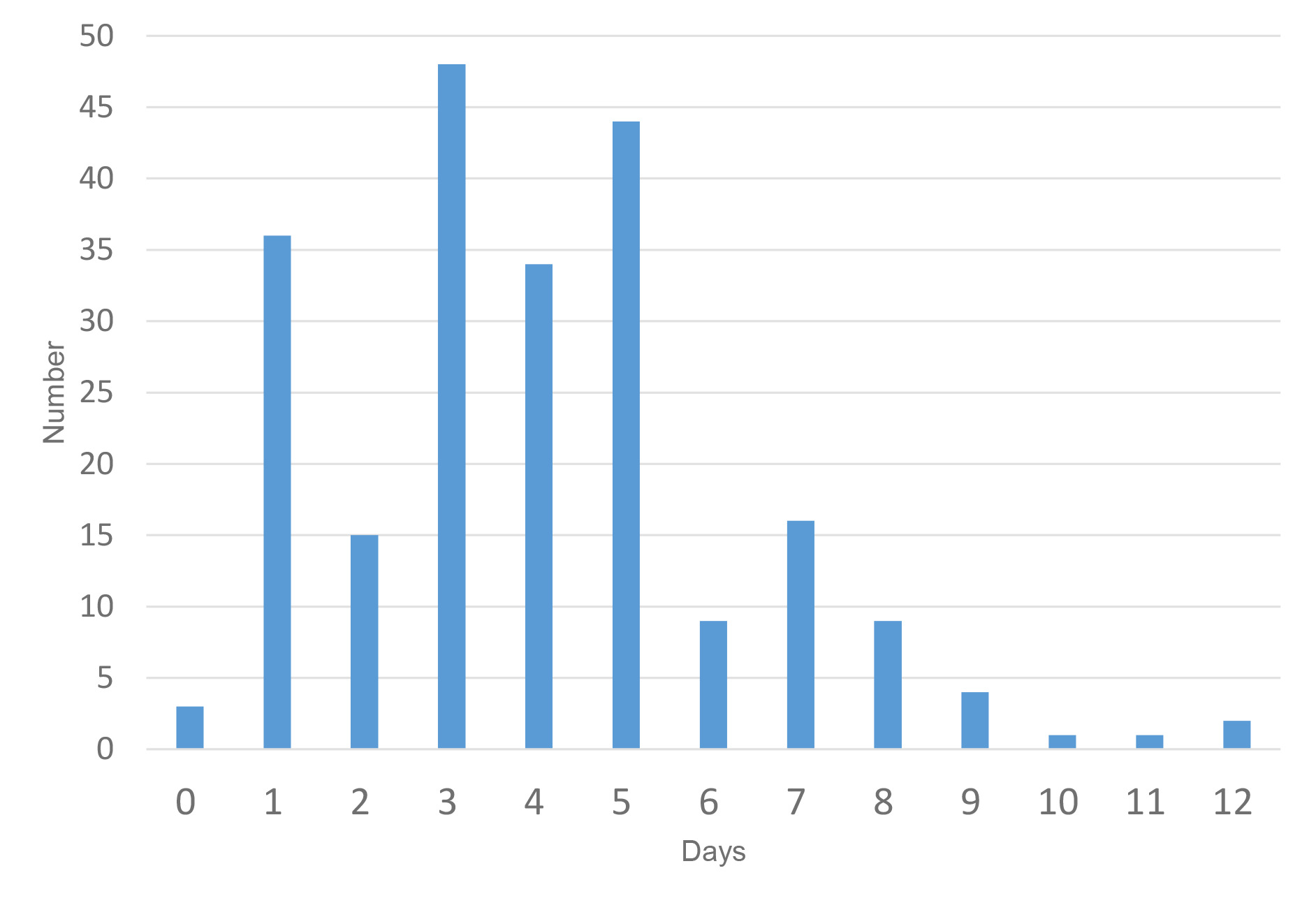
The duration of antibiotics prescribed ranged from zero to 12 days. The mean length of antibiotics used was 3.99 ± 2.29 days. The most common duration was three days (21.6%), followed by five days (19.8%) then one day (16.2%). We compared patients who received a ‘short’ (0–2 day) course of antibiotics with those who received a ‘long’ (3–5 day) course of antibiotics (Table 3). Factors associated with a long course of antibiotics were delay to first operation, excisional debridement and skin graft operations. Patients who had a scrub debridement and BiobraneTM (Smith+Nephew, Building 5, Croxley Park, Hatters Lane, Watford, Hertfordshire, WD18 8YE, UK) application operation tended to receive a short (0–2 day) course of antibiotics.
There was no difference between the rate of positive postoperative wound swab results and re-starting of antibiotics or commencement of new antibiotics between patients receiving a short or long course of antibiotics. There was also no difference in the rate of bacteraemia or antibiotic resistance (Table 4).
There were three cases of bacteraemia in this cohort (1.4%). Two out of the three cases of bacteraemia were related to a central line.
Discussion
This study was undertaken to audit our unit’s perioperative antibiotic prophylaxis practices and observe if the duration of antibiotics prescribed impacted clinical outcomes. Almost all (98.6%) acute burns patients requiring operative management received perioperative antibiotics, most of whom (86.8%) received cephazolin or cephalexin. The next most common antibiotic prescribed was piperacillin and tazobactam. Those who received piperacillin and tazobactam were significantly older (58.3 ± 19.6 versus 45.3 ± 17.8, p = 0.02) than those who received cephazolin or cephalexin. Although not statistically significant, patients receiving piperacillin and tazobactam tended to have a longer time from injury to first operation [5 (2.5–10) versus 2 (1–5), p = 0.06] and were more likely to have cultured an organism from a preoperative wound swab (0.47 versus 0.26, p = 0.08). These findings suggest that our practice was to prescribe cephazolin or cephalexin to most patients and piperacillin and tazobactam to older patients with delayed presentations and contaminated wounds, presumably for improved gram negative and anaerobic coverage. This was in keeping with our unit’s policy. Burn wounds become colonised by gram-positive bacteria first, followed by gram-negative bacteria from the environment, patient’s gut or naso-oropharyngeal tract over the next few days.1 This supports our practice of extending the antibiotic coverage for delayed and contaminated wounds. While a larger per cent of total body surface area (TBSA) burns are associated with early gram-negative wound colonisation in our unit,16 our results did not demonstrate use of broader antibiotic coverage for more extensive burns. Some patients, particularly those with delayed presentations and requiring excisional debridement, may have received perioperative antibiotics as both treatment for an existing wound infection and prophylaxis for further surgical site infection. Unfortunately, the retrospective chart review was not able to clearly delineate established infection, developing infection or clean burn wound, so all perioperative antibiotics used in this study were considered for the purpose of surgical site infection and bacteraemia prophylaxis.
There was a variable distribution of duration of antibiotics prescribed with duration ranging from zero to 12 days. We compared patients who received a short course of 0–2 days of antibiotics with those who received a long course of 3–5 days of antibiotics. At our institution, it is routine to examine postoperative wounds and decide upon need for further antibiotics by day five. It may be that further use of antibiotics beyond this time point was for treatment for infection rather than prophylaxis and excluded them from our analysis. The two groups for comparison were decided upon due to the distribution of antibiotic duration demonstrated in Figure 1 with three peaks at days one, three and five. Patients with a longer time from injury to first operation [1.5 (1–4) versus 3 (1–6), p = 0.03) were more likely to receive a long course of antibiotics. Patients who had a larger per cent TBSA burn [7 (3–14) versus 4.5 (1.5–7), p < 0.01] and per cent TBSA debrided [6.3 (2–12), 3 (1–7), p < 0.01] tended to receive a shorter course of antibiotics. The depth of burn may have confounded this result as those who had a scrub debridement and Biobrane™ application operation (0.39 versus 0.17, p < 0.01) were also more likely to receive a short course of antibiotics. These results likely represent more judicious use of antibiotics for more superficial burn wounds, even if they were larger in size, that can be managed with Biobrane™ dressings. On the other hand, patients requiring skin grafts received a longer course of antibiotics (0.31 versus 0.68, p < 0.01). A study performed by Ramos and colleagues demonstrated that prophylaxis use reduced graft loss in acute burns patients.17 However, the mean post-burn day for grafting in their study was 24 days, suggesting that grafts were laid on already colonised wounds. Overall, the evidence for prolonged courses of antibiotic prophylaxis to prevent graft failure is not strong in either burns or plastic surgery literature.7,8,18–20 Our study did not examine graft survival or the need for re-grafting as this outcome was not documented clearly enough to enable meaningful analysis on retrospective review.
Across many surgical specialties, prolonged courses of antibiotics are not superior to a single dose of antibiotics at induction for preventing surgical site infections.15,21 This is also the case among plastic and trauma surgery patients.19,20,22 Despite early studies demonstrating a reduction in wound infection and bacteraemia rates from antibiotics compared to placebo,6–8 no trials comparing single and multiple doses of antibiotics for prophylaxis in acute burns surgery exist. Our study displayed no difference in the rate of wound infection between a short and long course of antibiotics. As this study was performed retrospectively, wound infection was defined using surrogate measures. These proxy measures have their drawbacks and possibly underestimate the presence of wound infection. To accurately assess this outcome, a prospective measurement organism count would be needed through wound biopsy and monitoring for clinical evidence of infection.7 Nevertheless, our results contest the need for multiple doses of antibiotics to reduce wound infection rates. This is particularly the case among minor burns patients where the indication for use of perioperative prophylaxis is already uncertain.23,24
While use of antibiotic prophylaxis does reduce the incidence of bacteraemia in burns patients,7 our study did not demonstrate a difference in bacteraemia rates between a short and long course of antibiotics. The rate of bacteraemia in our cohort was 1.4 per cent, far lower than previously described rates of 12.5 per cent following wound manipulation procedures.25 In addition, two out of the three cases of bacteraemia in our cohort were related to central lines, suggesting that rates of bacteraemia may be lower than previously thought. Mozingo and colleagues also demonstrated that bacteraemia rates were relatively low in minor burns patients and questioned the need for antibiotic prophylaxis in patients with less than 40 per cent TBSA burns.25 This risk of bacteraemia does increase with more extensive burns, later postburn procedures, heavily colonised or infected wounds and aggressive surgical procedures.5 Risk stratification is likely needed in order to delineate which patients necessitate longer courses of antibiotics. In all likelihood, multiple doses of antibiotics are not required for the majority of burns patients.
There was no difference in rates of antibiotic resistance identified during admission or up to three months post discharge between a short and long course of antibiotics. Increased use of antibiotics is thought to correlate to increased risk of antibiotic resistance. This study only recorded the length of antibiotic use surrounding the first operation, not the total number of antibiotic days, a measure which has been associated with increased prevalence of multiple-drug resistance organisms among burns patients.14 Detection bias may have also played a role in this finding. Many patients in the long duration group were discharged home postoperatively with a course of five days of cephalexin and may not have had a follow-up wound swab to detect development of antibiotic resistance.
Limitations
Our study had several limitations largely due to its retrospective nature. Surrogate measures were used to record wound infection, which potentially misrepresented the true rate. Using wound swabs as a component of the definition for wound infection may have overestimated the true rate of infection by introducing detection bias for simple wound colonisation rather than infection. On the other hand, wound infections treated on the basis of clinical evidence of an infection without a positive wound swab result would have been under-represented in our study results. This is because clinical evidence of infection differentiating wound colonisation and wound infection was often not clearly documented, making it difficult to identify the indication for prolonged and further use of antibiotics. Another limitation due to the retrospective nature of this study was the inherent differences in the groups prescribed short and long courses of antibiotics, such as per cent TBSA burn, days to surgery post injury and type of operation. These factors were not able to be controlled for and may have confounded the lack of difference observed in wound infection and bacteraemia rates. A prospective trial is required to definitively assess whether a difference exists between short and long courses of antibiotics among burns patients.
Conclusion
The results of our study demonstrate variable use of perioperative antibiotic prophylaxis within one burns unit. Some factors such as age, time from injury to operation and the type of operation performed appeared to influence the form and duration of antibiotic prescribed. However, there were many cases of unsubstantiated use of long courses of antibiotics without apparent benefit on clinical outcomes of wound infection or bacteraemia. In the current setting of improved wound infection control, early debridement and wound closure, the need for perioperative antibiotic prophylaxis for all burns surgeries is called into question. With the growing concern over antibiotic overuse and development of resistance, there is an increasing need for development of clear guidelines for antibiotic use in burns surgery. This would likely require a trial comparing single and multiple doses of prophylaxis with prospective monitoring of clinical outcomes of wound infection, bacteraemia and graft failure.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship, and/or publication of this article.