Introduction
Excess submental fat (SMF) creates an obtuse cervicomental angle detracting from facial aesthetics and has been the target of facial rejuvenation with surgical and nonsurgical reduction methods.1 Liposuction offers treatment of SMF and provides consistent results.2 In patients who do not elect to undergo a surgical procedure, ATX-101 (Belkyra® Allergan, Longphort House, Block J, Leeson Street Lower, Saint Kevin’s, Dublin 2, D02 NY60, Ireland) offers a minimally invasive SMF reduction method. Previous nonsurgical methods of SMF reduction used products without robust scientific evidence for safety and efficacy and often provided only limited improvement.3,4
High resolution surface scans of the submental area offer a previously undescribed quantitative assessment of the efficacy of treatment, while the FACE-Q survey provides a validated tool to assess patient outcome satisfaction.
We present our treatment results for four consecutive patients who underwent injection of ATX-101 for submental fat reduction.
Methods
Prior to treatment and six weeks after the conclusion of the treatment period clinical photographs and high resolution 3D surface scans were taken of each patient’s face and neck area. For the 3D surface scans a portable high resolution structured light scanner (Artec Eva®, Artec3D, 20 Rue des Peupliers, 2328, Luxembourg) was used. All scans were performed with the patient seated in a dentist chair with hip flexion at 45 degrees to create a reproducible position. Patients were asked to fix their gaze on a consistent point and put their teeth into occlusion, without clenching, to create a reproducible degree of cervical flexion for pre- and post-treatment scans. All 2D and 3D images were recorded by a single researcher. This provided standardised soft tissue alignment conditions for scans pre-and post-treatment.
The presence of sufficient submental fat was confirmed with clinical examination prior to treatment following the manufacturer’s recommendation. Inclusion criteria for participation in this study were: adults aged < 65 years of age, body mass index (BMI) < 30, no previous significant medical conditions and no previous operations in the area of interest.
All injections were performed by the senior surgeon, as per the protocol supplied by the manufacturer. The superior border of the treatment area was 1.5 cm inferior to the border of the mandible, as marked from the angle to the mentum, with the thyroid notch delineating the inferior border. The injection grid template provided with each vial of ATX-101 was then used to mark injection sites that were spaced 1 cm apart. The amount of injecting points was then determined by addressing all grid template marks that fell into the treatment area. Pre, intra and post-procedural application of an ice-pack to the area was performed to minimise swelling and bruising and to reduce pain. Belkyra® was provided in single use vials of 2 mL solution of 10 mg/mL concentration. As per the manufacturer’s protocol, each injection point was injected with 0.2 ml treatment solution equaling 2 mg ATX-101 per treatment point. Care was taken to insert the 30 gauge needle perpendicular to the skin into the preplatysmal fat pad. No local anaesthetic was used as this is not included in the description of technique offered by the manufacturer.
Informed written consent was obtained from all patients. The study was approved by the Prince of Wales Clinical School, University of New South Wales ethics committee [HC180698].
Results
Patient one underwent three sessions of treatment with 24, 20 and 25 injections of 0.2 mLs in the three sessions. The total volume injected was 13.8 mLs. Patient two received four sessions of treatment with 23, 20, 22 and 20 injections of 0.2 mLs. The total volume injected was 17 mLs. Patient three received four sessions of treatment with 25, 21, 24 and 22 injections of 0.2 mLs in the four sessions, with a total volume of 18.4 mLs given. Patient four underwent four sessions of treatment with 22, 23, 25 and 20 injections of 0.2 mLs in the four sessions of 18 mLs of ATX-101 in total. Apart from injection site pain at the time of treatment there were no significant adverse reactions or complications in the four patients treated in this case series. Table 1 displays patient demographics, the number of treatment sessions and dosages.
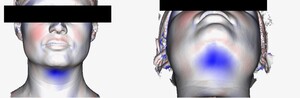
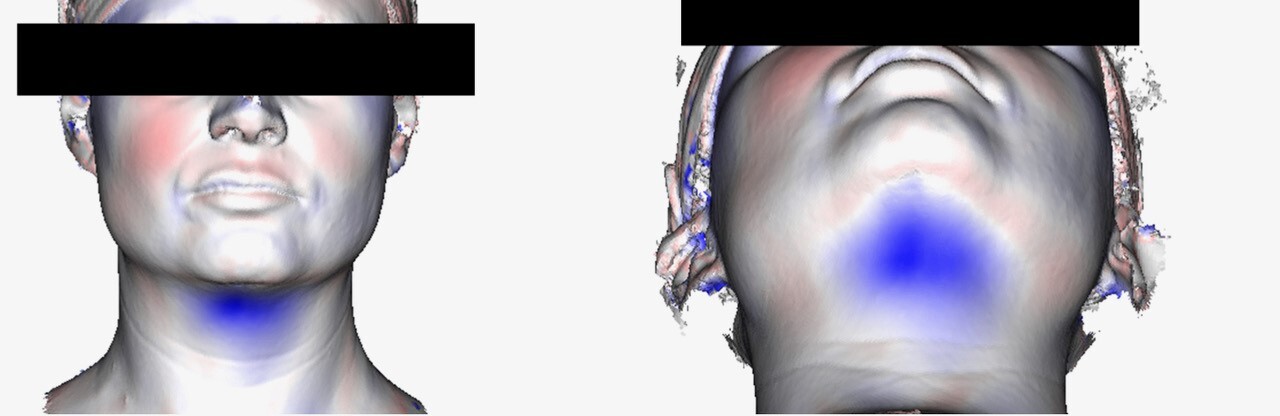
Imaging was pre-treatment and six weeks post- final treatment. From each scan a 3D mesh of each patient’s surface contour was generated providing a 0.1 mm point accuracy (0.03% accuracy over 100 cm) as shown in Figure 1. The pre- and post-treatment scans were then superimposed and the contour differences compared for each patient. Further algorithmic processing of the scans generated a cross sectional cut in the midline sagittal plane (Figure 2), representing the patients profile. The pre-treatment cross section was designated red and post-treatment cross section green. The maximum sub-mental projection difference was then measured in millimetres.
Table 2 demonstrates the reduction in submental fullness, equitable to a reduction in submental fat pad thickness. Patient one had 2.5 5 mm reduction in submental fat pad profile, patient two had 3.2 mm, patient three had 5.8 mm, patient four had 4.8 mm reduction. The mean reduction in submental profile was 4.09 mm (± 1.5 mm). The patients had no change in body mass index (BMI) from the onset to conclusion of the study period.
Patient satisfaction with the treatment was assessed with a FACE-Q patient survey. The survey measured three parameters of the treatment: subjective appraisal of submental area (fullness, skin laxity, and contour) pre- and post-treatment, satisfaction with the decision to undergo treatment and satisfaction with the outcome of treatment (Table 3). The sum of the scores was converted to an equivalent RASCH transformed score (0–100, with 0 the worst and 100 the best) using the FACE-Q conversion table. All patients subjectively noticed an improvement in their submental area, and the overall mean satisfaction with decision and outcome was 65.6 out of a possible maximum score of 100.
Discussion
Current minimally invasive fat reduction techniques include cryolipolysis, thermal lipolysis modalities (ultrasound, radiofrequency) and low-level laser therapy as well as chemical lipolysis. Cryolipolysis uses controlled cold exposure with natural thermal diffusion to induce apoptotic fat cell death and reduce the subcutaneous fat layer.5 In two studies, Coolsculpt® (Allergan, Longphort House, Block J, Leeson Street Lower, Saint Kevin’s, Dublin 2, D02 NY60, Ireland) has been demonstrated to be effective in reducing submental fat.6,7 Non-invasive radiofrequency (RF) delivers deep heating to adipose tissue for fat reduction. A single centre prospective study of 21 patients assessed the efficacy and safety of TruSculpt® (Cutera Inc, 3240 Bayshore Blvd, Brisbane, CA 94005, United States) for submental fat reduction, demonstrating statistically significant reduction in submental circumference and thickness.8 However, pain during RF-assisted contouring can be difficult to manage.9 High intensity focused ultrasound (HIFU) lipolysis works by convergence of high-energy ultrasonic waves at a target point.10 A prospective study of 20 patients evaluated the efficacy and safety for submental fat reduction and showed satisfactory improvement.11 Low-level laser therapy targets cytochrome C oxidase and evacuates fatty contents of the cell into the interstitium, that is then excreted by the body.12 A study of 30 patients demonstrated reduction in small volume focal areas on MRI with associated skin tightening.13
ATX-101 is a synthetic deoxycholic acid derivative which emulsifies cell membrane phospholipids and cause cytolysis with particular selectivity for low protein tissue such as adipocytes. An inflammatory process involving neutrophils and macrophages then follows which clear cellular debris and oil.14 As it is a cytolytic process, the effect of ATX-101 on adipocytes is irreversible. The efficacy and safety of ATX-101 has been extensively studied in multiple international phase I, II and III trials. Phase I trials showed ATX-101 to have no significant effect on the cardiovascular system, nor on the plasma concentration of total cholesterol, triglycerides, free fatty acids, or proinflammatory cytokines.15,16 Further phase II and III trials confirmed that there were no adverse systemic complications associated with use of ATX-101. Localised reactions to ATX-101 in phase III trials17,18 included haematoma (53.7%–70%), pain with injection (65.4%–80.2%) and firmness at injection site (18.3%–26.4%). Self-resolving marginal mandibular nerve paresis (4.3%) were reported in the two REFINE trials as well as one case of superficial skin ulceration.17,19 A case report of skin vascular occlusion event from deoxycholic acid injection has been reported in the literature.19 In the REFINE-1 phase III trial by Jones and colleagues 75 per cent of patients attained objective improvement of at least one grade in clinician reported submental fat rating scale. In addition, quantitative MRI in this study demonstrated subjects injected with ATX-101 versus placebo solution had a statistically significant reduction in SMF. They were also eight times more likely to have demonstrable reduction of SMF on MRI (46.3% vs 5.3%, p < 0.001) but it was still less than half of the subjects.
We present our experience with four consecutive patients who underwent injection of ATX-101 for submental fat reduction performed according to manufacturer’s recommendations. Our findings quantify the proposed results of ATX-101 on submental fat pad reduction in regards to the surface contour. Prior to treatment each of these patients had preprocedural PPG (pinch and palpate, pull, grimace) clinical assessment of their preplatysmal fat in the submental area, excluding other potential causes of submental fullness, as described by the manufacturer.
The use of high resolution surface scan provides objective and quantitative evidence of the efficacy of ATX-101 in irreversible adipocytolysis. Artec Eva® (Artec3D, 20 Rue des Peupliers, 2328, Luxembourg) has been validated in the literature as a reliable and reproducible 3D imaging method and is equivalent to other widely accepted 3D technology such as Vectra 3D® (Canfield Medical Imaging, 343 Passaic Ave, Fairfield, NJ 07004, United States).20,21 We were able to demonstrate a mean improvement in submental profile when comparing pre- and six weeks post-treatment scans. Many of the publications demonstrating efficacy have been clinical trials affiliated with the manufacturer and our independent study adds non-affiliated data. There were no major adverse reactions noted in our case series. Multiple studies have previously demonstrated the long-term efficacy of ATX-101 due to its irreversible effect on adipocytes,22,23 we anticipate the fat reduction seen in our patients should be sustained in the long-term too but our data only represents six weeks post-treatment results. Post-treatment FACE-Q data also demonstrated a trend for positive patient satisfaction. We recognise that the limitation of this study in terms of the limited cohort number and length of follow up, and present it as an initial experience to be expanded upon with time.
Conclusion
We demonstrated a mean reduction in submental fullness of 4.09 mm (± 1.5) and quantifiable patient satisfaction with the treatment. We conclude that ATX-101 can be a useful treatment for patients with excess submental preplatysmal fat who do not desire a surgical intervention.
Prior publication
Paper presented at Australasian Society of Aesthetic Plastic Surgery annual meeting, 21st October 2017, Melbourne, Australia.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Disclosure
The authors have no financial or commercial conflicts of interest to disclose for any of the products, devices, or drugs mentioned in this case series.
Funding declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Revision dates: October 21, 2021 AEST