Introduction
Sweet’s syndrome is a rare neutrophilic dermatosis characterised by eruptions of painful, erythematous plaques, elevated inflammatory markers, and pyrexia.1–5 Management is medical rather than surgical, and the wounds typically heal without scarring.5
We present an unusual case of Sweet’s syndrome involving one surgical site but sparing another in a patient undergoing simple skin excisions.
Case
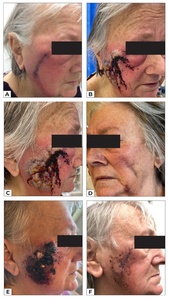
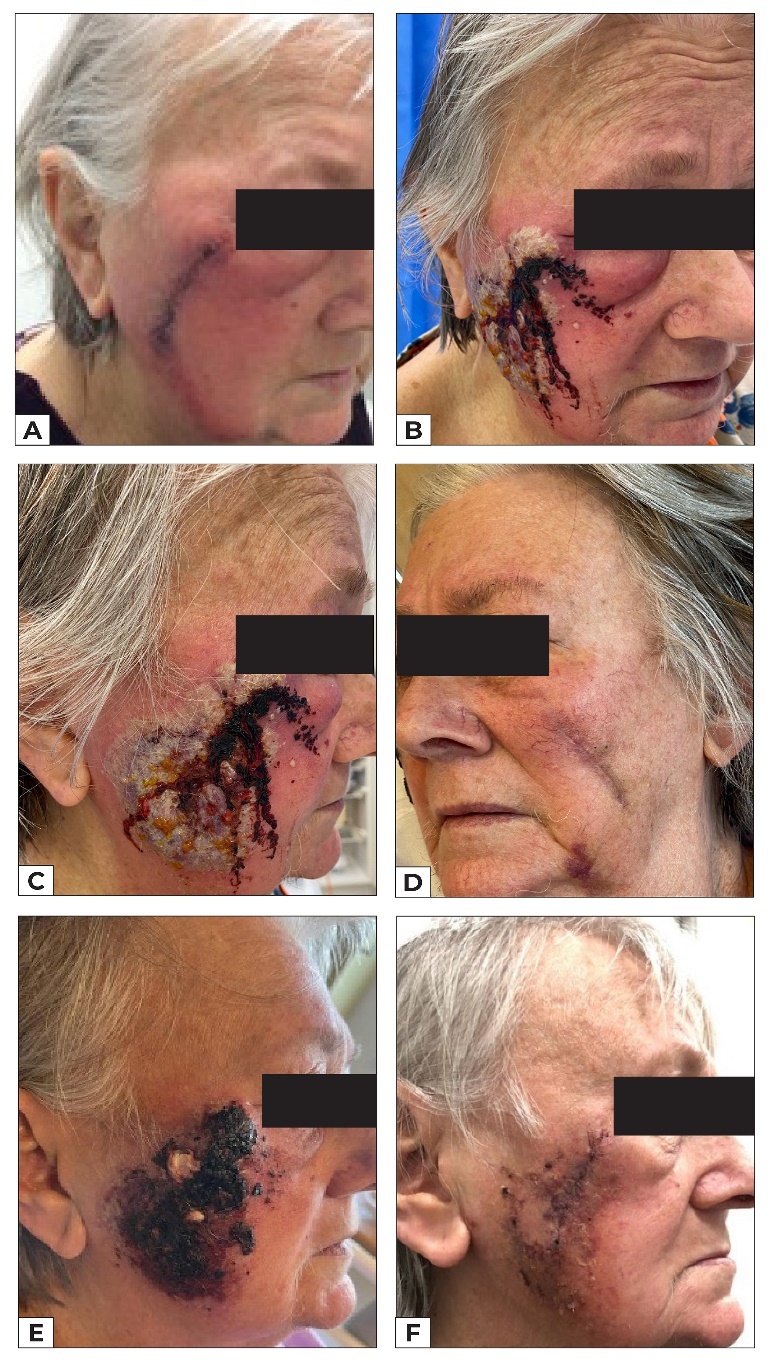
An 80-year-old woman underwent excision and direct closure of bilateral cheek skin lesions. On the third day postoperatively, the patient developed significant erythema, pain and swelling around her right facial surgical wound (Figure 1a). A diagnosis of cellulitis was made, and the patient was commenced on oral antibiotics. By day five postoperatively, the wound had significantly deteriorated despite treatment, with worsening local inflammation, painful plaque formation, and discharge from the right cheek wound, prompting presentation to the emergency department (Figures 1b and 1c). Despite her impressive wound, she had been systemically well with no constitutional symptoms. The left-sided facial excision site was unaffected. Her previous medical history was significant for type II diabetes mellitus (diet-controlled), atrial fibrillation on apixaban and metoprolol, hypertension on irbesartan and hydrochlorothiazide, hypercholesterolaemia on rosuvastatin, and stage IV chronic kidney disease (CKD). She is a non-smoker and lives independently in the community with her husband. On examination, the left cheek wound was clinically unaffected (Figure 1d). Her vital signs and serum white cell count were within normal limits (8.6*109/L), but serum C-reactive protein concentration was elevated (167mg/L). A wound swab grew Staphylococcus aureus. Despite the appearance of the wound, the absence of systemic symptoms led to a suspicion for a neutrophilic dermatosis.
She was admitted to hospital and the infectious diseases and dermatology teams were consulted. A tissue biopsy revealed an epidermal ulcer with dense suppurative inflammatory cell infiltrate of predominantly neutrophils, and a provisional diagnosis of Sweet’s syndrome was given (Figure 2). She was commenced on 50 mg oral prednisolone, antibiotics and corticosteroid therapy. After 24 hours of treatment, the patient improved dramatically and was discharged on a weaning dose of prednisolone (Figure 1e). At 12 days postoperatively, the inflammatory plaque was almost completely resolved (Figure 1f). At three months following the excision, she had healed with a good cosmetic result (Figure 3). Malignancy screening blood tests performed at the time of admission were negative and therefore the underlying trigger for Sweet’s syndrome in this case was likely infection.
Discussion
Sweet’s syndrome, also known as acute febrile neutrophilic dermatosis, is a rare inflammatory condition originally described by dermatologist Robert Sweet in 1964.1,2 It is a reactive condition characterised by painful erythematous plaques, dense dermal neutrophilic infiltrate, and a dramatic response to corticosteroid administration.1,3 It predominantly affects women and shows no racial disparity. Sweet’s syndrome may be recurrent in up to one-third of patients.4 The usual course of illness involves a preceding non-specific systemic illness followed by an active phase of dermatological and/ or extracutaneous involvement, with variable resolution of symptoms without treatment. The two major diagnostic criteria are an abrupt onset of tender erythematous plaques with vesicles, pustules or blisters, and histological evidence of dense neutrophilic dermal infiltrate in the absence of leukocytoclastic vasculitis—both should be present to make the diagnosis.3,5
Classification of Sweet’s syndrome is according to its association with other conditions or insults. Classic Sweet’s syndrome is considered idiopathic but also encompasses cases associated with infection, pregnancy, and inflammatory and autoimmune disorders.2 Malignancy-associated Sweet’s syndrome has been reported in patients with both haematologic and solid tumours, and drug-induced Sweet’s syndrome is most commonly associated with granulocyte-colony stimulating factor.3,5
Management of Sweet’s includes removing or treating the underlying cause (if known) along with medical therapies. Systemic corticosteroid therapy is the gold standard treatment, and this may be supplemented by topical steroid use. Other first-line agents include colchicine and potassium iodide. Of importance, surgical debridement does not improve Sweet’s syndrome and may in fact lead to worsening of the condition.6
Surgical trauma has been implicated in a handful of published case reports.7–9 In all the reported cases, the surgical insult was much greater than in this case.8,9 One of the patients had a known history of Sweet’s syndrome. In two of three cases, the patients developed symptoms in a similar timeframe to this case (within two to three days postoperatively). In the only case with multiple surgical sites, all surgical sites were affected, though the manifestations at the abdominal donor site lagged behind those seen at the reconstructed breast by several days.9 Interestingly, in this case the patient only had unilateral involvement, with the left cheek spared of any reaction despite a similar surgical insult. Early surgical site infection of the right cheek wound may have precipitated the unilateral Sweet’s syndrome, though there is no way to confirm this.
Conclusion
This case is a reminder for surgeons to consider Sweet’s syndrome as a differential diagnosis when a patient presents with a severe postoperative wound complication, or unusual wound. The clinical appearance in this case could easily be mistaken for necrotising soft tissue infection. Similar to other neutrophilic dermatoses such as pyoderma granulosum and Behcet’s disease, Sweet’s syndrome is associated with cutaneous pathergy, such that operative management would have precipitated significant worsening of dermatosis-associated lesions.
Acknowledgement
Thank you to Dr Sooraj Pillai, Pathologist at Gold Coast University Hospital for their valuable role in diagnosis of this patient’s condition, as well assistance in providing and interpreting the histopathology slides.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship and/or publication of this case report.