Introduction
Ovine Johne’s disease is a chronic wasting disease of sheep and goats resulting from Mycobacterium avium subspecies paratuberculosis infection. The mainstay of management in endemic flocks in Australia is Gudair vaccination. Unintentional human inoculation with Gudair can cause severe tissue reaction.
Published literature advocates for early surgical clearance of the vaccine to prevent the development of granuloma and necrosis but has yet to demonstrate this to be a viable treatment approach. Our series details seven patients who have been successfully managed with intentional delay to definitive debridement post-Gudair inoculation and provides grounds for clinicians to question the existing management rhetoric.
Background
Ovine Johne’s disease (OJD) is an incurable, wasting disease affecting sheep and goats. The manifestations result from a chronic enteritis caused by Mycobacterium avium subspecies paratuberculosis (MAP) infection.1–3 Bacterium spread between animals typically occurs through the faecal-oral route.4 Mortality has been measured as up to 17.5 per cent.5 Ovine Johne’s disease was first identified in Australia in 1980. The disease has since been isolated in sheep or goats in most Australian states.5
There is currently no cure for OJD; the mainstay of management in endemic flocks is vaccination.4 Gudair (CZ Vaccines O Porriño, Pontevedra, Spain) is the only OJD vaccine registered for use in Australia.6 The vaccine does not provide absolute protection against MAP infection, or mortality from OJD.4,6 The vaccine-stimulated cell-mediated humoral immune response produced by Gudair has, however, been found to delay MAP shedding and reduce the prevalence of shedders and clinical disease.6,7
Gudair contains inactivated MAP cells in combination with a mineral oil adjuvant.8 The adjuvant is a crucial contributor to the efficacy of the vaccine, acting as a potent immune stimulator.4 Mineral oil vaccines are typically considered too reactive for human use.3 The vaccine is administered subcutaneously, high on the neck of the sheep. When delivered as prescribed, a firm swelling will typically appear at or near the inoculation site after 7–15 days.6,7 Injection site lesions persist in up to 50 per cent of vaccinates at two months and remain in 20–25 per cent at four years.6,8 An abscess forms at the site of the lesion in five per cent.1,6
Human inoculation with Gudair can cause severe tissue reaction, with granuloma formation and necrosis far greater than that typically observed in sheep.3 The following case series outlines the management and clinical course of seven patients who sustained unintentional inoculation with Gudair vaccine. These patients presented between 2009 and 2021 and underwent surgical management by a single consultant plastic and reconstructive surgeon. They represent the complete cohort of Gudair-inoculated patients treated by the surgeon.
Case series
Case 1
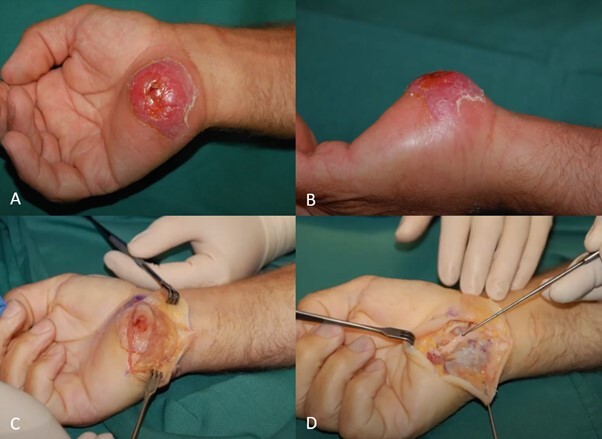
56-year-old man, inoculation right hand
The patient presented to his general practitioner (GP) on the day of injury and was given a steroid injection. The following day oral antibiotics were commenced. Surgical review occurred on day four, where mild swelling to the hypothenar base was noted. Elevation, cooling and anti-inflammatories were prescribed. One-week post-inoculation, a weeping granuloma was apparent. The need for debridement was documented but intervention intentionally delayed until 29 days post-inoculation to allow for stabilisation of the tissue reaction.
Radical debridement of the granuloma was performed, with partial excision of the palmaris brevis. Primary closure of the wound was achieved. The patient returned to work with a glove for protection within a week. At 35 days post- debridement, the surgical site was healed and the hand comfortable and fully functional. No further surgical intervention was required. (Figure 1).
Case 2
39-year-old man, inoculation left hand
The patient commenced oral antibiotics prescribed by his GP on the day of injury. Local swelling developed and anti-inflammatories were commenced on day three. Three weeks post-injury, a persistent swelling of the site was lanced by the GP and discharge of ‘yellow custard’ was noted. Surgical opinion was sought five months post- inoculation. The region of the left first webspace was found to be broadly indurated.
Thorough debridement of granulomatous tissue was undertaken. This extended over the free edge of adductor pollicis to the base of the proximal phalanx of the thumb. Primary closure was achieved. There were no complications at review six weeks post-debridement. No further surgical intervention was required.
Case 3
30-year-old woman, inoculation right posterior-medial knee
The patient presented to her GP on the day of injury, where the injection site was lanced and irrigated. She was commenced on oral antibiotics. The patient presented for surgical opinion 14 weeks post-inoculation. A draining, multiloculated granulomatous lesion of the posterior-medial knee was apparent.
Radical excision of an ellipse of skin and the underlying granulomatous tissue was performed the following week. The excision site was closed primarily in layers. There were no complications at review eight days post-excision and no further surgical intervention was required. (Figure 2).
Case 4
49-year-old man, inoculation left medial knee
The patient’s region of injury rapidly became erythematous, swollen and painful. The patient experienced transient improvement with commencement of oral antibiotics before development of further swelling and a mass lesion.
Surgical review occurred three weeks post- inoculation and the patient proceeded to excision the subsequent day. An ellipse of skin was removed along with an underlying abscess cavity. The excision site was closed primarily over a drain. There were no wound concerns at review 16 weeks following surgery. No further surgical intervention was required.
Case 5
51-year-old woman, inoculation left dorsal forearm
The patient attended a regional hospital three days post-inoculation. Diffuse swelling of the distal dorsal forearm was noted. Oral steroids and antibiotics were commenced. Surgical review occurred one month later, before the patient proceeded to resection of a caseating granuloma of the forearm. The granulomatous tissue was found to extend along the tendons and tissue planes. The surgical site was primarily closed.
One-week post-debridement, she presented to the public hospital and proceeded to theatre for drainage of a haematoma. At five weeks post- debridement, healing remained incomplete and her forearm was re-explored. A region of necrotic skin was excised, along with granulation tissue that had formed between tendons. No further granulomatous tissue was identified. The wound was again primarily closed. At two weeks following the exploration, the patient was able to return to work. No further surgical intervention was required.
Case 6
27-year-old woman, inoculation left knee
The patient was formally reviewed two months post-injury following multiple local attempts at drainage of the inoculation site. Two weeks later, the patient underwent surgical excision of skin and an underlying purulence-containing granuloma which extended into the quadriceps. The surgical site was primarily closed. At one-week post-excision, there were no complications and the patient returned to work. No further surgical intervention was required.
Case 7
39-year-old man, inoculation right distal volar forearm
The patient was initially admitted to a regional hospital and commenced on intravenous antibiotics. He presented for surgical review two weeks later, where the proximal volar forearm was found to be swollen, indurated and tender. The patient declined to undergo debridement following some improvement to pain and movement.
One-month post-inoculation, erythema and warmth of the forearm increased. Antibiotics were recommenced and the patient agreed to surgery six weeks post-inoculation. A region of skin breakdown was excised in addition to extensive debridement of the flexor compartment. Primary closure was achieved. There was appropriate healing and good range of motion at review one week following surgery. No further surgical intervention was required. (Figure 3).
Discussion
As Gudair vaccine contains inactivated MAP, there is no risk of live bacterium transmission in human inoculation. Environmental contamination of hypodermics used in animal vaccination may, however, contribute to secondary bacterial infection in unintentional human inoculation.2 The role of prophylactic antibiotics should be considered and tetanus status evaluated.8,9 The florid tissue response observed in humans is thought to primarily result from reaction to the deposited vaccine, rather than infection. The use of antibiotics will not prevent the development of granuloma or necrosis in human Gudair inoculation.8 The only proposed method of preventing necrosis is through surgical clearance of the vaccine.
Literature discussing the management of human Gudair inoculation consistently advocates for early operative intervention to remove the vaccine prior to development of the granulomatous reaction.1–3,8,9 This recommendation comes despite an absence of case reports demonstrating successful early, single-operation debridement and primary closure post-inoculation. Furthermore, no author has attempted to define the window in which ‘early’ debridement should occur.
Ex vivo Gudair vaccine is white in colour.1 This appearance is reportedly initially maintained in vivo (personal communication) but within days the vaccine material may no longer be macroscopically identifiable within tissues.1 No histologic stain presently exists to accurately differentiate vaccine mineral oil from human oils, to aid in establishing adequacy of clearance.3 Any attempt to explore an inoculation site and remove vaccine material before it has been localised by the immune reaction is hypothesised by the authors to contribute to further manipulation of the vaccine along tissue planes, where the tissue reaction will later inevitably manifest.
The case histories reported here represent the largest published Australian series managed by a single surgeon. Six of the seven patients who sustained Gudair inoculation were successfully managed with a single operative debridement with primary closure. The one patient who required a return to theatre was noted to be a heavy smoker and comorbid, with poor compliance to postoperative instructions. In each case, even with early presentation, operative intervention was intentionally delayed until the anticipated tissue reaction had stabilised and the vaccine components were localised within the tissues.
Conclusion
In our experience, we recommend first aid for unintentional human Gudair injection that includes gentle cleansing of the injury site without milking or massage, administration of tetanus booster if indicated, empirical oral antibiotics targeted to likely needle contaminants, oral anti-inflammatories such as corticosteroids, and consideration of the role of splinting or immobilisation.
We believe that the development of a granulomatous reaction is inevitable and is unlikely to be prevented through early attempts at drainage of the vaccine unless formulation changes occur to facilitate in vivo identification. Definitive surgical debridement of necrotic and granulomatous tissue should only be performed once the lesion has declared itself and stabilised. This, together with meticulous dissection and broad resection margins, will facilitate safe primary closure, minimise the need for repeated return to theatre and unanticipated complications to wound healing. Through this approach we have demonstrated comparatively early and definitive return to work post-debridement. In addition, the burden on the healthcare system through multiple theatre attendances is avoided and the economic impact of lost productivity on the patient is expected to be reduced.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship and/or publication of this article.