Introduction
In Australia, approximately 12,600 coronary artery bypass grafts are performed annually.1 Deep sternal wound infections occur in 0.2–3.0 per cent of cases.2 Pedicled flaps, which are the workhorse reconstruction option, are straightforward to harvest with relatively short operative times, but suffer limitations such as their arc of rotation, vulnerability to partial flap necrosis, limited utility in reconstructing larger defects and may not be an option in salvage cases.3 In the setting of free flap reconstruction of sternal defects, finding suitable recipient vessels around the sternum can be challenging, especially when the left internal mammary artery has been used for cardiac revascularisation. In the scenario where left internal mammary artery has been used for cardiac grafts, most surgeons hesitate to use the right internal mammary artery nd vein as recipient vessels due to concerns of sternal devascularisation.3 This often necessitates using recipient vessels in the base of the neck and the use of either vein grafts to increase pedicle length or arteriovenous loops in a staged fashion.3,4
Case series
Case I
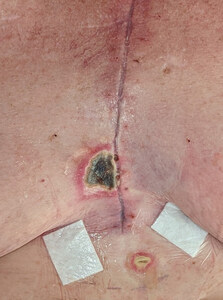
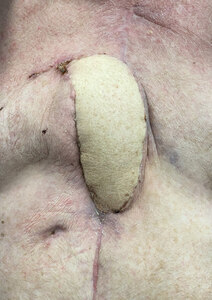
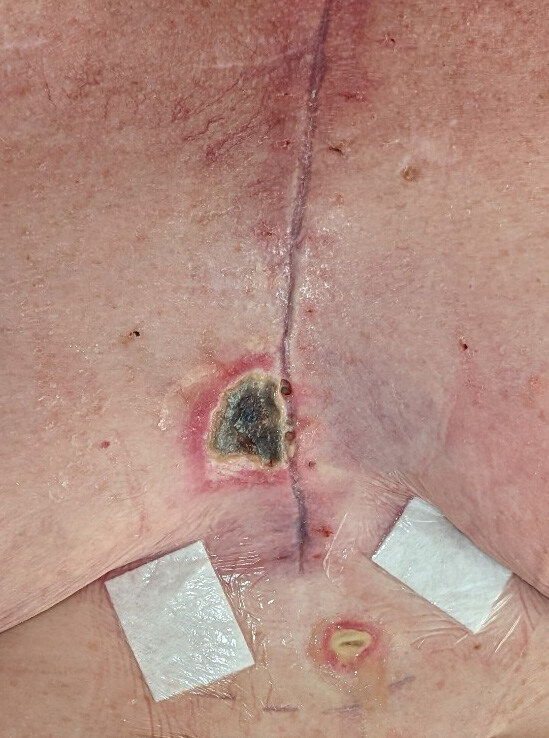
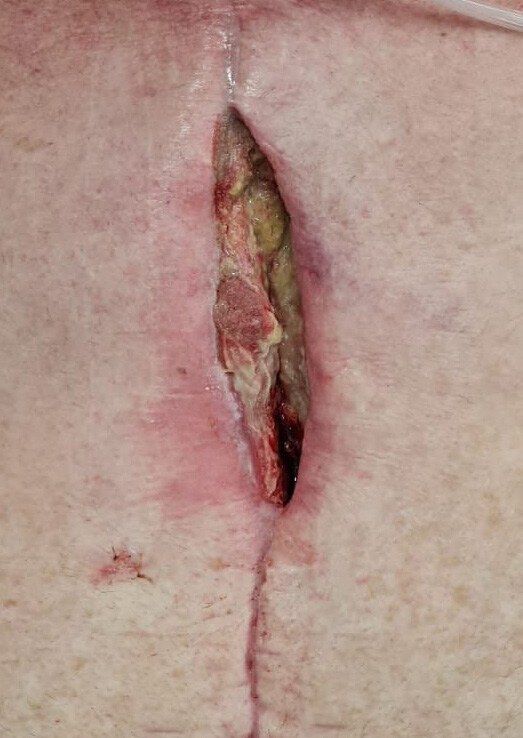
A 56-year-old woman with a background of diabetes and obstructive sleep apnoea underwent triple cardiac bypass surgery following myocardial infarction. Her left internal mammary artery (LIMA) and great saphenous vein (GSV) were harvested. She presented three weeks post-coronary artery bypass graft (CABG) with a deep sternal wound infection (DSWI). The wound was serially debrided, sternal wires removed, and a vacuum-assisted closure (VAC) dressing was used to temporise the wound. Intraoperative swabs grew Klebsiella pneumoniae and Enteroccoccus faecalis and the patient was treated with amoxicillin-clavulanic acid as guided by the infectious disease team. Before the patient was referred to our unit, the sternal defect was reconstructed with bilateral pectoralis major advancement flaps which failed. Further debridements were performed conjointly between the plastic surgery and the cardiothoracic surgery teams resulting in a 3 x 3 cm defect over the inferior third of the sternum requiring soft tissue cover. Once negative swabs were obtained, the sternal defect was covered with a fasciocutaneous anterolaterial thigh (ALT) free flap. For the recipient vessels, a window in the right third rib was created to access the right internal mammary artery (RIMA) and right internal mammary vein (RIMV). An end-to-end arterial anastomosis was performed between the descending branch of the lateral circumflex femoral artery (DBLCFA) and RIMA using 8-0 nylon. An end-to-end venous anastomosis was performed between the venous comitante of the DBLCFA and RIMV using a 3 mm venous coupler. The flap was inset with 3-0 Monocryl and 4-0 Prolene sutures. The patient progressed uneventfully postoperatively and completed four months of antibiotic therapy. She was completely healed at one-year follow-up. Figure 1 shows the sternal wound preoperatively. Figure 2 demonstrates the postoperative result at one-year follow-up.
Case II
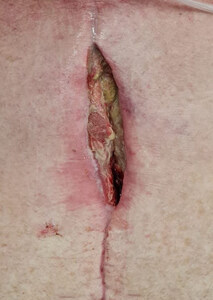
A 60-year-old man with a background of diabetes, obstructive sleep apnoea, previous stroke, and heavy smoking, underwent triple cardiac bypass surgery using the LIMA, GSV and left radial artery. He presented five weeks later with a DSWI associated with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia. Guided by the infectious disease team, the patient was treated with antibiotic therapy which included vancomycin, linezolid, amikacin and ceftazidime according to the cultures that grew MRSA, Pseudomonas aeruginosa and extended-spectrum beta-lactamases (ESBL). Before the patient was referred to us, he underwent bilateral pectoralis major advancement as well as omental flaps to cover the sternal defect but this was complicated by wound infection and dehiscence. Further debridements were performed conjointly between the plastic surgery and cardiothoracic surgery teams. The residual defect measured 2 x 7 cm over the middle third of the sternum. We performed an ALT free flap using the same technique and methods of recipient vessel preparation as for case I. The patient progressed uneventfully and completed long-term rifampicin and fusidic acid antibiotic therapy. He was completely healed at his six-month follow-up. Figure 3 shows the sternal wound preoperatively. Figure 4 demonstrates the postoperative result six-months follow-up.
Discussion
Sternal vascular anatomy
The internal mammary artery (IMA) arises from the first part of the subclavian artery and travels down behind the medial end of the clavicle.5 It descends 1–2 cm from the lateral sternal border before bifurcating into the superior epigastric and musculophrenic terminal branches.6 During its course, the IMA gives four types of branches including the intercostal, perforating, mediastinal, and sternal branches. Sternal branches arise opposite each interchondral space, pass medially towards the sternum, and bifurcate into the anterior and posterior periosteum. These vessels collateralise in the periosteum with branches above, below and on the contralateral side to form a dense plexus. Arterial blood supply of the sternum is derived solely from this periosteal plexus fed by segmental branches of the IMA. There is no medullary nutrient system to supplement sternal perfusion.5,6 Thus when the IMA is dissected for use as recipient vessels in microsurgery, branches should be ligated as close as possible to the main vessel to preserve the collateral branches to the sternum.7
Preserving sternal perfusion with internal mammary artery grafts
There are longstanding concerns that bilateral IMA harvest for CABG reduces sternal perfusion and predisposes the patient to DSWI.7 The hesitancy in using the RIMA/RIMV as recipient vessels for free flaps in the setting of LIMA harvest stems from this notion.3 Authors have proposed that the risk of DSWI using bilateral IMA grafts for CABG can be mitigated by harvesting a ‘skeletonised IMA’.7,8 This involves harvesting the IMA without its accompanying vein, nerves and endothoracic fascia and ligating the branches close to their origin.8 This skeletonised technique of harvesting the IMA is not dissimilar to the way recipient vessels are prepared for free flaps. Berdajs and colleagues in their dissection studies of the IMA found that sternal blood supply was best preserved by collaterals from the anterior intercostal arteries when the IMA branches were ligated as close as possible to the main vessel.9 Boodhwani and colleagues randomised 48 patients undergoing CABG with bilateral IMA harvest to receive one skeletonised and one non-skeletonised IMA.7 They assessed sternal perfusion intraoperatively with a Doppler and found that sternal perfusion was 17 per cent greater on the skeletonised side.7 Similarly, Cohen and colleagues randomised 24 patients to receive a skeletonised versus non-skeletonised left IMA harvest and found 20 per cent greater sternal perfusion for skeletonised IMA which was measured objectively using pre- and postoperative bone scans.8 According to Shin and colleagues the risk of sternal devascularisation and DSWI can be further mitigated with atraumatic vessel handling and meticulous dissection to preserve sternal blood flow.10
Furthermore, the retrograde flow of IMA and IMV has been successfully used as the donor vessel in numerous free flap reconstructions.11 Thus after using RIMA as recipient vessels for free flaps, it may be possible for the distal IMA to still perfuse the sternum in a retrograde fashion. In our two cases, the recipient RIMA were skeletonised by ligating branches close to the origin, preserving collateral connections for sternal vascularization.
Free flaps for sternal reconstruction
Sternal defects following DSWI are predominantly reconstructed using loco-regional flaps. However, free flaps are increasingly used for sternal reconstruction with case series using the ALT, TFL, latissimus dorsi (LD), vastus lateralis, and vertical rectus abdominis musculocutaneous (VRAM) free flaps.3,4,12,13
Wee and colleagues reconstructed 20 sternal defects using the extended ALT free flap and found that patients who underwent free flap reconstruction had significantly less donor site complications (seroma, breast distortion, skin graft loss) compared to patients who underwent pedicled flap reconstruction (pectoralis major and LD) (21 % vs 0 %).3 Falkner and colleagues found that pedicled flaps for large defects were associated with a higher rate of partial flap necrosis and additional revision operations were often needed.13 Wee and colleagues used the transverse cervical artery/external jugular vein, thoracoacromial artery/cephalic vein, superior thyroid artery/anterior jugular vein as recipients, and cited concerns that the IMA may not be effective in CABG patients with IMA grafts.3
Bigdeli and colleagues reconstructed 46 sternal defects using the tensor fascia lata (TFL) free flap.4 Similar to our cases, a significant proportion of patients underwent TFL free flap reconstruction due to failed attempts with pedicled flaps. Recipient vessels used were the RIMA and RIMV in 20 per cent of patients, and the RIMA and cephalic vein in 33 per cent. However, it was not mentioned if these patients had intact LIMA/LIMV systems. For the remainder of their patients, neck recipient vessels were used and arteriovenous loops were necessary.4
In our experience the ALT flap has several advantages. The flap can be harvested supine and the vastus lateralis can be included for additional bulk. Even though muscle flaps are traditionally used in areas of infection, both our patients with severe sternal osteomyelitis had no recurrent infection and demonstrated complete wound healing at long-term follow-up. To the best of our knowledge, these are the first cases of sternal reconstruction describing the use of the RIMA/RIMV as recipient vessels in the setting of LIMA harvest. Unlike the neck vessels, the RIMA system is located within the operative field for sternal reconstruction. The dissection is also familiar to many plastic surgeons who use the same recipient vessels in breast reconstruction.
Conclusion
Although pedicled flaps are traditionally used for sternal reconstruction, free flaps should be considered for larger defects and salvage cases where pedicled flaps have failed. In the setting where left internal mammary artery is absent, our series adds to the literature regarding the safe use of the right internal mammary artery and right internal mammary vein for end-to-end anastomoses in these settings. The risk of sternal devascularisation can be further mitigated by atraumatic vessel handling and meticulous dissection with minimal stripping of sternal branches.
Patient consent
Patients have given informed consent to the publication of images and/or data.
Funding declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
The authors have no conflicts of interest to disclose.
Revised: August 8, 2023