Introduction
Hypothenar hammer syndrome (HHS) is a rare condition that was first described by Conn and colleagues in 1970.1 Incidence in the general population is reported as only 1.17 per cent, while estimates in industrial settings are as high as 14 per cent.2,3 Hypothenar hammer syndrome usually affects the dominant hand, with risk factors including manual labour and tobacco smoking. Clinical diagnosis is based on findings of arterial insufficiency which usually presents with a combination of digital or hypothenar pain, cold intolerance, phasic blanching or fingertip discolouration.4 Ultrasound is typically performed as an initial diagnostic investigation.5 Gold standard imaging modalities are contrast computed tomography angiography (CTA) or magnetic resonance angiography (MRA).
Non-surgical management of HHS includes lifestyle modification (smoking cessation, avoidance of cold) and medications aimed at improving blood flow to the extremities (calcium channel blockers and prostaglandins).2 Indications for surgical treatment are failed non-operative management and/or critical digital ischaemia. Principles of surgical management include addressing the underlying vascular insufficiency (thrombectomy, resection, reconstruction) and debridement of devitalised tissue.2,3 Percutaneous interventions such as localised thrombolytics and balloon angioplasty have been reported to be effective in acute presentations, however, in the absence of randomised control trials or large case control studies, they do not show superior outcomes compared to surgery in acute or chronic presentations.5,6
Surgical reconstruction of the ulnar artery with venous grafting is a well-documented procedure with relative surgical ease, a variety of potential donor sites and low donor site morbidity.7,8 Despite these benefits, venous grafts are not optimally suited to the pressures of the arterial system and are susceptible to graft failure through occlusion and dilation. Arterial donors adhere to the reconstructive principle of replacing ‘like for like’. Due to its calibre and branching pattern, the deep inferior epigastric artery (DIEA) is well suited to reconstruct the ulnar artery in HHS, but is an infrequently reported donor.9
We describe a useful and infrequently reported donor option for autologous like-for-like surgical reconstruction of the ulnar artery in HHS using a DIEA graft in two cases.
Case 1
A 42-year-old right-hand-dominant male labourer was referred to the plastic and reconstructive surgery team with worsening right middle finger pain and pulp necrosis. He was an active smoker (20 pack year) with a history of intravenous drug use. He had presented to the emergency department several times over the previous three months with no cause for his pain identified. He reported multiple bouts of spasmodic pain, exacerbated by cold weather and partially relieved by holding his hand in a dependent position. Prior to review, he had been admitted under the general medical team for four days and had received intravenous pain relief via a patient-controlled ketamine analgesia device. Consults by rheumatology and vascular surgery were unable to identify a cause for his pain. On examination, he had a pulsatile mass in the hypothenar region, hypoaesthesia of the dorsal middle finger and necrosis of the middle finger pulp. Allen’s test was normal at the wrist and digit.
Biochemical markers were within normal limits. Screening X-ray, ultrasound and CTA showed no abnormalities. Targeted, high resolution CTA and MRA identified a short area of marked tortuosity and 6 mm diameter aneurysm to the ulnar artery as it passed to the superficial palmar arch. The common digital artery to the middle finger was not seen beyond its origin at the superficial palmar arch (Figure 1). An aortic CTA identified a patent uncalcified DIEA as a suitable donor for reconstruction.
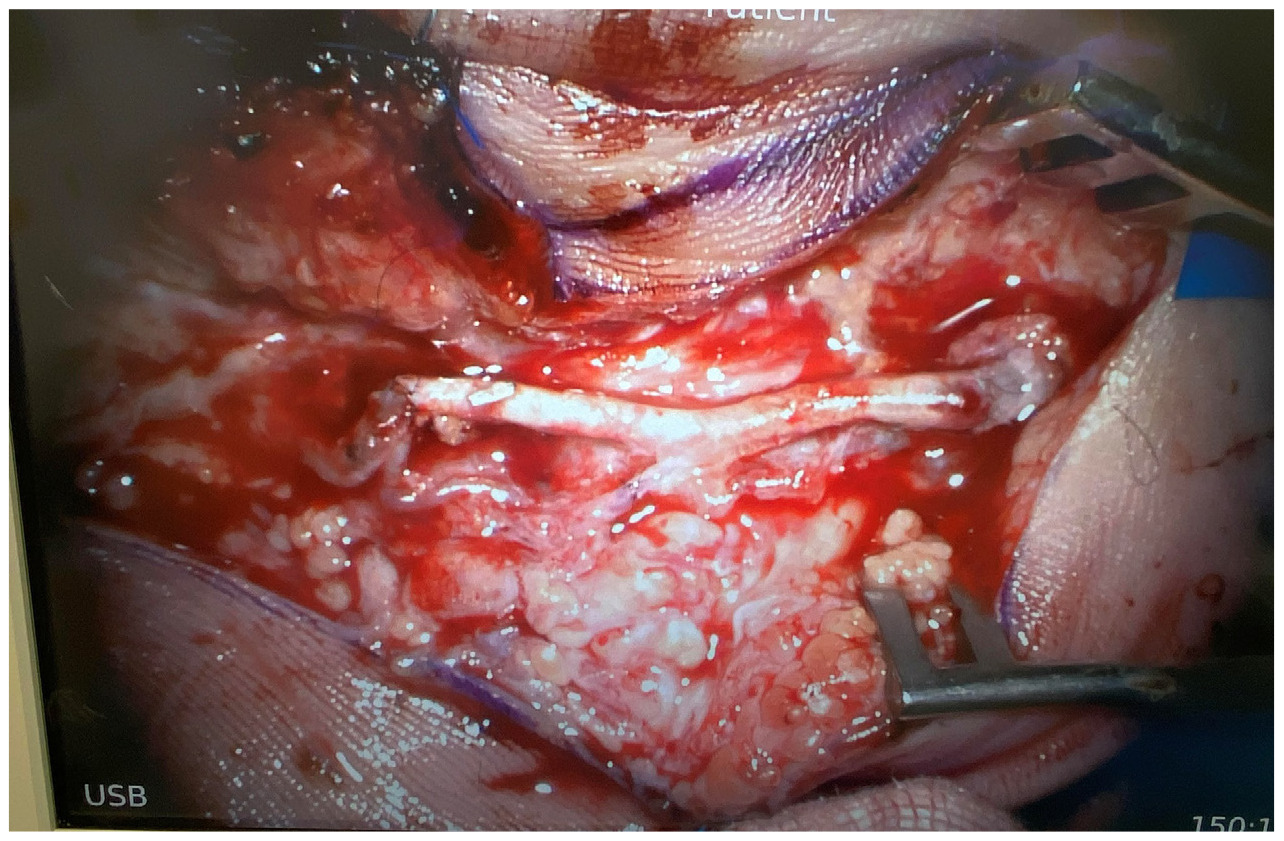
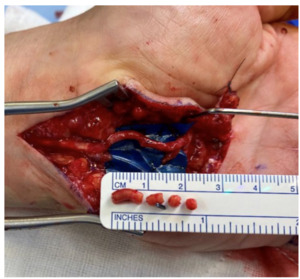
The patient underwent surgical exploration, where the aneurysm and tortuous portion of the vessel was identified and serially excised until adequate flow was seen through the palmar arch. This resulted in a 2.6 cm arterial defect, which included the origin of the palmar digital artery to the little finger (Figure 2). The DIEA was harvested through a groin incision and exposed to select for adequate size including branches available to replicate the palmar arch. This was used as an arterial interposition graft to replace the resected segment of palmar arch as well as the palmar digital artery defect (Figure 3). Following closure of the surgical wound, the necrotic tip to the finger was debrided back to healthy tissue. Histopathology of the resected ulnar artery showed a large muscular blood vessel with evidence of vessel wall damage and intra-luminal thrombosis. The patient recovered well and was discharged on day two postoperatively without complication and minimal oral analgesia requirements.
At his one-week postoperative review the patient reported resolution of the hypoaesthesia and pain. His two-week review revealed a small amount of skin necrosis over the previously debrided middle finger requiring further debridement with shortening of the exposed distal phalanx. This skin necrosis was thought to have been caused by underdebridement in his initial procedure, but may also have occurred as sequelae of the initial debridement in a vulnerable tissue bed. At six weeks all wounds had completely healed and he had returned to full time manual work. No issues were identified at his three- or six-month reviews.
Case 2
A 51-year-old, right-hand-dominant retired male fitter and turner was referred to the plastic and reconstructive surgery team with a six-month history of spasmodic pain and pulp necrosis to his left little finger. He had a past medical history of rheumatoid arthritis and hypertension and had recently undergone a cubital tunnel release on the ipsilateral limb at another hospital. He required regular long and short acting breakthrough opioid analgesia to control his worsening pain. He was an active smoker (20 pack year).
On examination, he had a necrotic and swollen left little fingertip with prominent callus over the hypothenar eminence. Firm palpation identified a tender mass at this position. Hypoaesthesia was present over the entire little finger. Allen’s test was normal at the wrist and digit. Biochemical markers were within normal limits, including inflammatory and autoimmune tests. X-ray of the left hand was unremarkable. Ultrasound showed a 25–30 mm length of occlusion commencing at the pisiform bone. A targeted CTA of the left hand showed thrombosed pseudoaneurysm of an occluded ulnar artery at the level of the hook of hamate. An abdominal CTA was performed which identified patent, uncalcified DIEA vessels.
The patient underwent surgical exploration, where the aneurysm and tortuous portion of the vessel was identified and serially excised to strong inflow and acceptable backflow through the palmar arch, with the resultant arterial defect being 2.5 cm. The DIEA with a suitable size match to the ulnar defect was harvested through a groin incision (Figure 4). It was then used as an autologous interposition graft and anastomosed under microscope magnification with 9-0 nylon (Figure 5). The necrotic tip to the little finger was debrided after closure. Histological examination of the ulnar artery revealed a large muscular blood vessel with luminal occlusive thrombus and the vessel wall largely replaced by fibrous tissue with foamy histiocytes.
The patient was discharged day one postoperatively with no opioid analgesia required. At his two-week review he was pain free. At his four-week review he was noted to have a donor site infection and was returned to theatre for washout and debridement where a small volume subcutaneous collection was evacuated. He was started on oral antibiotics. At his six-week review he remained pain free, his wounds had healed, and his hand had returned to pre-morbid functionality. This benefit was maintained at his three- and six-month reviews.
Discussion
Hypothenar hammer syndrome can present as acute thrombosis or rarely intermittent thrombosis with chronically worsening symptoms. Furthermore, the ulnar artery at the wrist is the most common site of arterial aneurysms of the upper extremity.2 In retrospective trials, arterial grafts have been shown as superior to venous grafts in upper limb arterial vasculature reconstruction, however, these benefits must be offset against possibly greater donor site morbidity and more limited donor site options.7–10 Alterations in flow dynamics within venous grafts subjected to arterial pressures have been shown to result in intimal hyperplasia causing partial or complete graft occlusion in up to 50 per cent of reconstructions, as well as long term dilation.11,12 Utilising an arterial graft adheres to the reconstructive principle of replacing ‘like with like’ and provides a graft more suited to the high-pressure arterial system.
In these two cases the DIEA provided an excellent donor for reconstruction post segmental resection of the ulnar artery in HHS. Reconstructive surgeons are familiar with the anatomy of the DIEA due to the overlap with other common reconstructive procedures, allowing for speed and precision with graft harvest. Furthermore, the location of the donor site allows the graft to be harvested concurrently with the resection of the disease, reducing operative time.13,14 Finally, the DIEA provides an excellent size match and the branching patterns can be selected to restore more complex defects (DIEA diameter around 3.5 mm, ulnar artery around 3 mm in Guyon’s canal).15 The DIEA can reliably supply more than 12 cm of length, allowing for reconstruction of extensive defects where an extended tortuous segment of vessel can be anticipated.
Despite these benefits, donor site morbidity (which can include debilitating anterior abdominal wall weakness) may be higher compared to venous conduits, graft harvest may render future flaps based on the arterial system unavailable, more complex graft harvest compared to many venous grafts may lead to prolonged operative time, and there is no specific outcome data available for comparison with alternative options.7,8,11,12,14 A recent expert review assessing arterial grafts compared to venous grafts in microvascular reconstruction of hand vessels recommended against their routine use due to donor site morbidity and a lack of well designed, prospective studies showing their reconstructive superiority.10 While not yet widely available, synthetic conduits may also represent a superior reconstructive option in the future.
Conclusion
Hypothenar hammer syndrome can be effectively treated by surgical resection and reconstruction of the diseased ulnar artery. The primary aim of surgical treatment is to reduce symptomatology and tissue ischaemia. We report a reconstructive option for HHS with an autologous DIEA graft providing a ‘like for like’ ulnar artery reconstruction with flexibility in reconstructing the palmar arch by implementing branching patterns as clinically indicated.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: November 7, 2022; January 26, 2023 AEST
_mra_and_(b)_reconstructed_cta_showing_a_short_segment_of_marked_tortuosity_and_a_s.png)
_pre_and_(b)_post_excision.png)


_mra_and_(b)_reconstructed_cta_showing_a_short_segment_of_marked_tortuosity_and_a_s.png)
_pre_and_(b)_post_excision.png)