Introduction
Polyacrylamide gel (PAAG), known as Aquamid® (Contura Limited, London, United Kingdom, EC4A 1LB) in Australia, is a hydrophilic, non-toxic substance comprised of approximately 2.5 per cent polyacrylamide and 97.5 per cent water. Introduced in the 1970s as a soft tissue filler, PAAG quickly gained popularity for cosmetic procedures in several countries including China, Russia and Iran.1,2 However, due to concerns regarding side effects such as breast cancer and glandular atrophy, it was banned in the late 1990s.3,4 Complications arising from PAAG injections have been well-documented in countries where it was commonly utilised, mainly in breast augmentation which made up approximately 80 per cent of its use.5 Common presentations include lumps, pain, deformity, displacement and infections occurring in up to 18.3 per cent of recipients in some studies.2
Within Australian literature, there is currently no published data regarding PAAG fillers. Given the increasing population of Chinese immigrants, it is likely that complications will be seen more frequently within our healthcare system in the coming decades.6 This case series aims to highlight the presence of PAAG filler complications in Australia using two case studies with a discussion about symptoms, signs and best-practice management.
Cases
Case 1
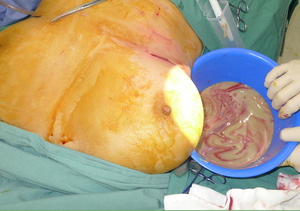
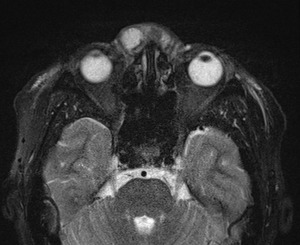
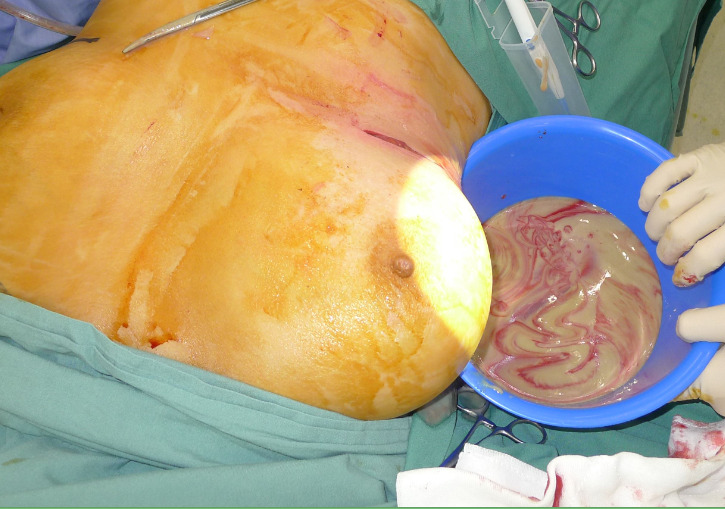
A 53-year-old woman presented with two months of right breast swelling and fevers with a background of bilateral free PAAG filler augmentation in China 14 years earlier. MRI showed free prosthetic material in subglandular and subpectoral pockets on both sides without parenchymal lesions (Figure 1). On examination she had marked swelling and mild erythema of the right breast with elevated inflammatory markers. The patient was taken to theatre the same day for evacuation of the prosthetic filler. Via bilateral infra-mammary incisions, 900 ml of purulent material was evacuated from the right breast (Figure 2) and 300 ml PAAG filler removed from the left breast (Figure 3). Tissue culture, swab culture and capsule histopathology demonstrated scant mix of skin flora. Mycobacterial PCR for tuberculosis was negative. Capsule histopathology from both sides showed fibrous pseudocapsule with extracellular foreign body material and a mixed inflammatory cell infiltrate. The patient completed a six-week oral antibiotic regime and made an uncomplicated recovery.
Case 2
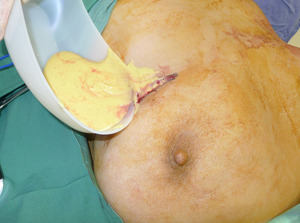
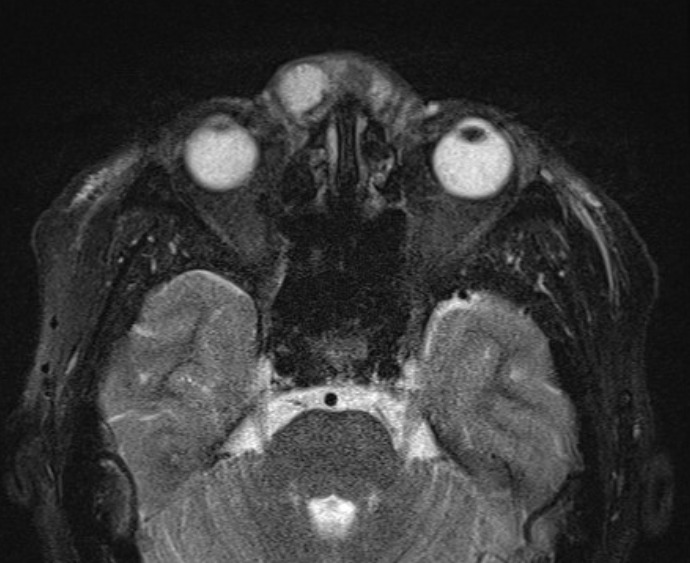
A 57-year-old woman presented with facial cellulitis with one week of increasing erythema and swelling of the nose, forehead and cheeks with a background of cosmetic nasal dorsum augmentation with PAAG filler in China eight years earlier. Ultrasound and MRI of the face showed two pockets of displaced filler material on the lateral aspect of the nose and left zygomatic arch. She was taken to theatre the following day to drain two abscesses on bilateral nasal side walls and to remove the PAAG filler (Figure 4). Swab for microscopy and culture did not isolate a dominant organism. Nasendoscopy showed no sinusitis or nasal discharge and healthy intranasal mucosa. The patient responded well to antibiotics and dressings and was discharged without issue.
Sample group
At least a dozen more patients have been seen by the senior author within the last two years with various symptoms and complications following PAAG filler injection. Some are awaiting elective surgical removal of the product while others have elected for observation only. Within this sample of patients, all presentations are for filler complications in the face or breast, with the initial procedure having occurred in China (Table 1).
Discussion
These cases demonstrate that PAAG filler complications are not uncommon within Australia, despite the fact that it was not widely used in Western countries outside of Europe.7 Our sample group focused on patients who had immigrated from China, where use of PAAG augmentation in the last 20 years was significant. Many of these patients had the filler injected over a decade ago. This suggests that the foreign material can lay dormant for many years before causing later complications. We believe this is the first study to focus on PAAG complications within Australia, highlighting the potential morbidity associated with a cosmetic procedure used extensively overseas that is relatively unknown to many local clinicians.
The aetiology of reactions to gel fillers is unknown, though Christensen suggests that they may be due to infection from commensal skin bacteria.8 In a study by Patlazhan, Staphylococcus epidermidis, either alone or with other organisms, was found in bacterial culture of the evacuated gel.9 De Boulle also describes a number of complication types from facial filler injection within European patients including: hypersensitivity, haematomas, pigmentation, necrosis and granuloma formation. These complications can occur by type I or type IV hypersensitivity reactions, or disruption to blood supply of or adjacent to the injected area.10
Treatment strategies
A number of articles investigating the best treatment for complications of PAAG breast augmentation have been published.11 Magnetic resonance imaging (MRI) is typically the most sensitive and comprehensive diagnostic modality, as it is able to clearly assess anatomical distribution of the foreign material. Ultrasound may also be helpful, while mammogram should be performed if there is suspicion of malignancy.11
Surgical removal of PAAG filler is the treatment standard, usually via a peri-areolar, inframammary or axillary incision.2,11–13 For displaced PAAG on the thoracoabdominal wall, Chen suggests using a sub-areolar incision combined with surgical drainage of the gel mass in the chest wall via an inferior approach, with suture closure of the fistula tract.14 Outcomes are usually satisfactory and most patients report resolution of symptoms postoperatively, as noted above in our first case study.2,9,11–13 Immediate breast reconstruction with silicone prostheses or autologous fat grafting can be offered for patients where there is no infection and the PAAG filler is completely removed. Alternatively, if there is any concern regarding infection or malignancy, delayed reconstruction should only be considered once the underlying issue has been treated, at least three to six months after initial surgery.2,11,12
Complications of facial augmentation can be treated with needle aspiration, incisional drainage or stab incision of the collection of PAAG filler with good effect.15 Radmanesh also describes a safe and effective technique using a fiberoptic laser by using the laser tip within a metal cannula to liquefy the material.16 However, in many cases, diffuse distribution of the gel and injection into multiple tissue planes can make treatment difficult or impossible, with unsatisfactory cosmetic outcomes for patients despite surgical intervention.17
Conclusion
In conclusion, late complications of cosmetic fillers can have significant morbidity and are not uncommon within the Australian healthcare system. Clinicians should be aware of PAAG filler as a potential differential diagnosis in patients who present with breast or facial soft tissue swelling, infection or deformity and have a history of cosmetic surgery, especially in immigrant populations from Asian or Eastern European countries. Treatment is generally surgical evacuation of the prosthetic material, although for smaller pockets such as on the face, needle aspiration or incisional drainage is an appropriate method of removal. Unfortunately, complete removal of the material is extremely difficult and patients should have ongoing follow-up with a plastic surgeon where possible.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Disclosure
The authors have no conflicts of interest to disclose.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Revised: July 5, 2018 AEST