Introduction
Pressure sores are a common complication in bed- bound patients, particularly in those immobilised due to spinal cord injury. There are estimated to be approximately 2760 patients living with spinal cord injury in Australia. The community prevalence of pressure sores in patients with spinal cord injury is approximately 23 per cent, making this a significant area of unmet clinical need.1,2
Vigilant pressure care and optimal nutrition remain the mainstays of prevention of pressure sores. However, pressure sores may be an inevitable consequence of restricted mobility and insensate weight-bearing bony prominences, particularly in areas of diminished padding due to muscular wasting. Recurrent ulceration and associated osteomyelitis often necessitate surgical debridement and long-term antibiotic treatment requiring protracted inpatient hospital stays. These admissions are often followed by ongoing dressing regimens in the outpatient setting. Once healed, unstable scar tissue makes the area prone to recurrent ulceration, and positioning the patient to avoid putting pressure on the affected area often results in synchronous pressure sores in other areas.
Reconstructing unstable scar and pressure sores requires transfer of robust, durable soft tissue from a non-weight-bearing donor site. Synchronous trochanteric and ischial pressure sores (the ‘dual- defect’), in particular, pose a difficult reconstructive challenge (Figure 1).
Numerous locoregional flaps have been described for the reconstruction of a greater trochanteric or ischial pressure sore but a technique for soft-tissue coverage of ‘dual-defect’ pressure areas has not been described.3 We sought a durable reconstructive technique for managing these concurrent pressure areas in spinal patients. The anterolateral thigh (ALT) flap has been described for each of these defects individually,4–6 however, we sought to apply the ALT flap for resurfacing the ‘dual-defect’ area. The ALT flap was originally described and modified by Chinese researchers in 19837 but was first described in English language literature as a free flap a year later.8 The 1990s saw a surge in the use of the ALT flap in China and Japan and this was followed by the publication of a Taiwanese series of nearly 700 cases in the early 2000s,9 after which the use of the ALT flap as a versatile workhorse fasciocutaneous free flap flourished.
The ALT flap is a mathes nahai type B (musculocutaneous) or type C (septocutaneous) flap and the vastus lateralis muscle is a type I mathes nahai muscle.10 The pedicle length is typically 12 cm with a pivot point approximately 10 cm below the anterior superior iliac spine (ASIS). Uses for this flap include head and neck reconstruction, upper and lower limb trauma and breast reconstruction in selected patients.10 Since coming to prominence as a mainstay of reconstructive microsurgery, however, publications describing the use of the ALT as a local pedicle flap have been comparatively sparse. The ALT flap boasts an impressive rotational arc, suitable for reconstructing defects including the anterior and posterior aspects of both the thigh and the inferior trunk, and the ipsilateral groin and ischium.10 We present and discuss a modification of the pedicled ALT flap for treatment of the ‘dual- defect’ pressure sore.
Methods
We undertook a prospective study of 11 appropriately selected consecutive cases. Spinal cord injury patients with concurrent pressure sores grade three or above, or unstable scar in one of the ‘dual-defect’ areas and an active pressure sore in the other, were included.
The cases were documented using clinical photography before, during and after surgery. Institutional consent for operative procedures and individual consent for participation in this study were obtained. Patient follow-up period was recorded. Operative details including operating time, on-table position and postoperative measures such as drain-tube placement and antibiotic administration were recorded prospectively. All complications were recorded and categorised as minor or major, according to a predetermined scale.
Operative technique
Flap design and markings
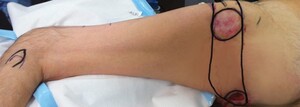
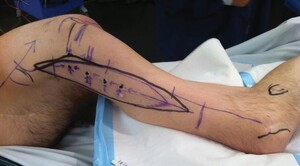
The ALT flap is designed around the descending branch of the lateral circumflex artery arising from the profunda femoris artery.8 Preoperative marking is undertaken using hand-held Doppler ultrasound (Figure 2). The ASIS (point A) and the fibula head are identified and a point 2 cm anterior to the fibula head is marked (point F). A line is drawn between the ASIS and point F (the A–F line). A point is marked 4 cm proximal to the midpoint of this line, denoting the location of the main perforator of the ALT. From this location, the point half-way to the knee and the ASIS is also marked. In ambulant patients, the perforators are said to be an average of 1.5 cm lateral to a line drawn from the ASIS to the patella11 but in spinal patients the soft tissues tend to fall anteriorly, bringing the line of the perforators in line with the A–F line in the mid-thigh region.
The anticipated defect is measured and marked over the greater trochanter and ischium and a template created. The arc of rotation is planned in reverse, individually for each defect, and marked on the lateral aspect of the leg, centred on the A–F line (Figure 2). The template of these defects is then positioned a further 2 cm distally on the leg, to account for length lost in flap transposition. The anterior and posterior borders of the flap are marked to correspond to the lateral and most medial points of the marked defects.
Raising the flap
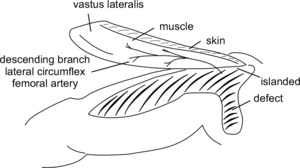
To raise a flap, the pressure sores are optimally debrided and washed and the final dimensions of the defects are measured and a template created. The pedicle length from the anticipated pivot point (approximately 10 cm below the ASIS) to the edge of the skin paddle is then re- measured intraoperatively. Initially the anterior incision is made, approximately 10–15 cm long, and continued down to the fascial layer, until the muscle fibres of the rectus femoris are visualised. At this stage it is important to verify the identity of this muscle (a task made more difficult in the spinal patient due to the adhered planes and atrophied musculature) because it is possible to commence the subfascial dissection too laterally and risk inadvertently dividing the critical skin perforators. Once the rectus femoris muscle has been identified, the fascia is incised over the muscle and the cleft between the rectus femoris medially and vastus lateralis laterally is followed down to the dominant pedicle (descending branch of the lateral circumflex femoral artery—LCFA), which is traced distally. The already non-functional motor nerve accompanying the vascular pedicle is ligated without consequence. Once again, care must be taken as tissues are adherent around the vessels and the pedicle is friable. Once the pedicle has been fully identified and mobilised, the skin paddle is defined and islanded to the subfascial level (Figure 3). The vastus lateralis may be included in the flap, and mobilised and divided at the distal musculotendinous junction, and an additional 1 cm extension of fascia lata is left distally.
Flap inset and postoperative management
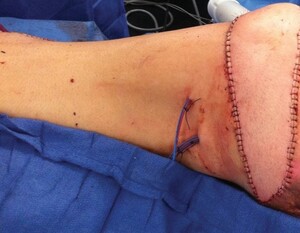
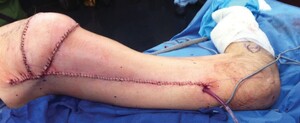
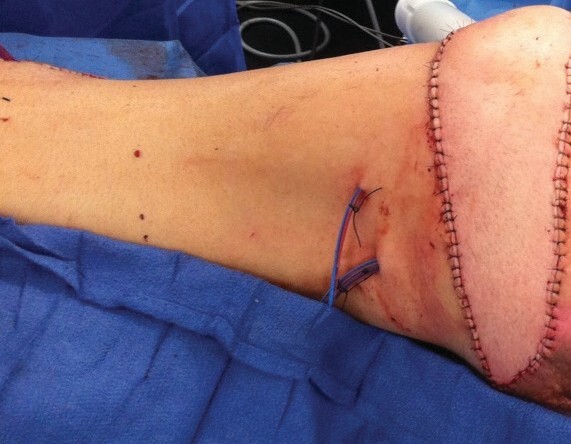
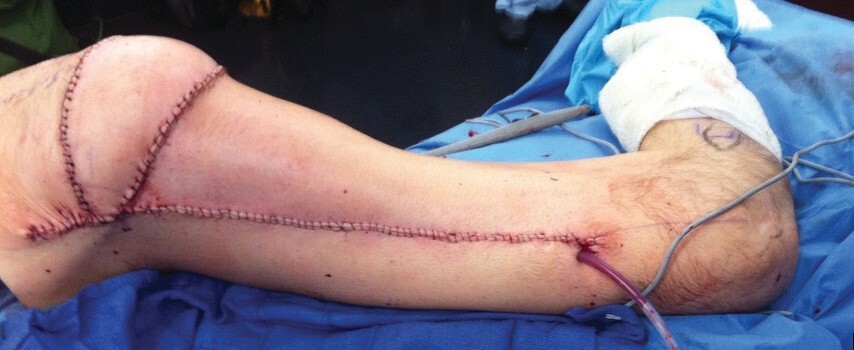
The distal tip of the flap is inset into the ischial defect with deep 2-0 vicryl parachuted stitches. The skin paddle is inset with 3-0 monocryl interrupted deep dermal and simple interrupted nylon sutures. One or two 10 Blake’s drain tubes are run along the length of the flap and a separate drain tube is run along the length of the thigh donor site (Figures 4 and 5).
Postoperative management consists of strict pressure care with no direct pressure on the flap or donor site for six weeks. Drain tubes routinely remain in situ for two weeks and cutaneous nylon sutures are removed after three weeks.
Results
The 11 recruited patients were aged between 34 and 73 years old at the time of surgery, averaging 48.2 years old. The cases were followed up for an average of 20.5 months. Measurements were taken for each of the pressure ulcer defects and the dimensions of the flap used to reconstruct these ‘dual defects’ were also recorded. The size of the trochanteric pressure sores was an average of 6 × 4 cm and the ischial pressure sores were slightly larger at 8.2 × 6.7 cm. The pedicled ALT flaps raised ranged from 19 × 9 cm to 33 × 8.5 cm, with average flap dimensions of 27.3 × 8.4 cm (Tables 1 and 2).
Seven of the patients experienced minor complications in the postoperative period. Five of these complications were wound infections that were treated conservatively with appropriate antibiotic cover and did not necessitate a return to theatre. Two patients were anaemic postoperatively (haemoglobin < 120 g/L) but did not require blood transfusion. There was one major complication, a donor site haematoma that necessitated return to theatre for washout. There were no unplanned readmissions.
Discussion
Pressure sores occur in nearly a quarter of patients with spinal cord injury in Australia.1,2 Many patients undergoing pressure care nursing for an initial defect in one of the common pressure areas suffer a second synchronous pressure sore in another area. Furthermore, one pressure sore, even when eventually healed, results in chronically attenuated or unstable skin that is susceptible to further metachronous episodes of breakdown in the future. Overall, this problem is not only morbid and, in some cases, life-threatening for these patients, but also an enormous resource and staffing burden for the healthcare system.
Trochanteric and ischial ‘dual-defects’ can be particularly troublesome in chronically bedridden or wheelchair-bound patients following spinal injury. Current commonly used techniques for reconstructing these defects include the V-Y advancement flap based on perforators of the superior or inferior gluteal arteries, the tensor fascia lata (TFL) flap, or the profunda-based posterior thigh or posteromedial thigh flap.
While researchers have previously described use of the ALT flap for reconstructing either trochanteric or ischial pressures sores, the use of a locoregional flap for ‘dual-defect’ reconstruction has not been described. Lee a described a new technique of transferring pedicled thigh and vastus lateralis myocutaneous flaps for reconstruction of recurrent ischial pressure sores in 2007, in which the flap was tunnelled through the thigh and inset into the ischial pressure sore posteriorly.6 In 2011, Kua described an islanded, pedicled ALT flap tunnelled via a lateral subcutaneous tunnel for recurrent ischial ulcers.4 Wang and Tzeng both described the reconstruction of trochanteric defects using a proximal pedicled ALT flap.5,12
Here we describe use of the pedicled ALT flap—a robust, simple musculocutaneous flap pedicled on the descending branch of the LCFA and the standard musculocutaneous or fasciocutaneous perforators of the ALT flap—for single-stage reconstruction of ‘dual-defect’ trochanteric and ischial pressure sores. This flap is not tunnelled and incorporates the entire vastus lateralis muscle, providing robust, vascularised muscle coverage of the bony prominences that may be chronically osteomyelitic, as well as resilient skin derived from a donor site that is usually well out of the typical ‘zone of injury’ of peri-pelvic pressure sores. Moreover, this flap is amenable to further advancement, if need be, and the donor site may be closed directly and is unlikely to be subject to pressure during future pressure care nursing. The addition of the vastus lateralis muscle to the cutaneous component of the ALT as a composite flap is without functional significance, as these patients are not ambulant. In addition, the subjective experience of the pain of dividing a muscle is not an issue and the issue of dysreflexia reported by spinal physicians with flaps in which the gluteus maximus muscle is divided does not seem to be as much of an issue.
The results of this study demonstrate the safety of this reconstructive method, particularly important in this already physiologically compromised patient cohort. Although two of the patients were anaemic in the postoperative period, none of the patients required administration of blood products and only one patient required a return to theatre. Minimising complications and returns to theatre is paramount, as these patients often already have protracted inpatient hospital admissions.13
Other advantages of this flap include the increasing familiarity of plastic surgeons with the anatomy of the ALT flap, making it quick and easy to raise. The flap can resurface dual trochanteric and ischial defects using healthy skin usually unaffected by pressure in chronic bedridden patients. The fascia lata cuff facilitates excellent watertight parachute inset into the defect. The use of the vastus lateralis facilitates easier donor site closure, allowing a wider skin paddle to be designed. Finally, a lack of any functional donor site morbidity in this patient group makes the pedicled ALT flap an ideal choice for resurfacing dual trochanteric and ischial defects.
It is our experience that several technical differences from the normally reliable soft-tissue anatomy and bony landmarks used in preoperative marking are important in this spinal injury patient population. We suggest several technical variations on the traditional description of the ALT flap to allow for altered anatomy in this patient group. Firstly, the anatomical landmark of the lateral border of the patella (and thus the A–P line) may be less valid than in the ambulant patient, as the patella is frequently subluxed. In our patient population we found a point that is 2 cm anterior to the head of the fibula (point F) is a more reliable marker of the true lateral aspect of the leg and recommend that the skin paddle be centred on this line (the A–F line) as the key landmark for the raise.
Secondly, the soft tissues are often atrophic and have slipped anteriorly around the convexity of the leg—this must be taken into account when marking the flap as perforators may arise anterior of the position that would be expected in the ambulant patient. This factor means that the preoperative Doppler ultrasound is of greater importance than in raising the ALT perforator flap for the ambulatory patient. In patients for whom the circulation of the thigh integument is medially dominated (these patients would normally be converted to an anteromedial thigh flap if an intended ALT flap was found to be devoid of adequate cutaneous perforators),11 the preoperative Doppler ultrasound detected a dominant cutaneous perforator from the transverse branch of the LCFA, through the TFL muscle, and the flap was extended superiorly to maintain continuity with this perforator in the skin paddle. In these cases the flap was not islanded superiorly.
Finally, it must be remembered that the quality of the tissues of long-term spinal injury patients is very different from the tissues of patients without spinal injury. In long-term spinal injury patients the skin becomes atrophic, the muscles become attenuated and friable and the tissue planes are therefore distorted. Extra care must be taken during flap dissection as tissue planes in this patient group are adherent and difficult to identify, while vessels are more friable and may be more difficult to discern than in the ambulant patient. The surgeon must question the safety of perforator dissection in unreliable tissue planes, and more robust pedicles, such as the descending branch of the LCFA, are much safer.
Conclusion
This study demonstrates that the pedicled ALT flap, with the technical variations described, is a safe and effective technique for reconstructing challenging ‘dual-defect’ pressure sores and can be viewed as a core element of our reconstructive armamentarium for spinal patients.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Revised: April 3, 2019 AEST