Introduction
Despite improvements in breast implant design and surgical technique, breast implant device infection and exposure remain a real concern following implant placement. Rates of infection in the literature have ranged from 0.4 to 2.5 per cent for augmentation mammaplasty, but are significantly higher, from 1 to 35.4 per cent, for prosthetic breast reconstruction after cancer.1,2 Loss of the implant and therefore the reconstruction can be devastating for patients with negative psychosocial impacts and even delays in oncological treatment.3
In common with international literature, our usual practice was the immediate removal of the infected or exposed breast prosthesis.4 An internal audit in 2015 of all implant infections from 2006 to 2014 at Middlemore Hospital, Counties Manukau District Health Board (CMDHB), Auckland, found that the Department of Plastic and Reconstructive Surgery inserted 218 prosthetic devices and had a 13 per cent infection rate (n = 28 implants) [data not published]. Of these 28 implants, 54 per cent (n = 15) required return to theatre for washout and 87 per cent (n = 13) of pockets washed out had implants removed due to severe infection, resulting in an overall 6 per cent implant loss rate.
In 2017, our department started using Veraflo vacuum-assisted closure (VAC) for infected lower limb wounds, as was well reported in the literature at the time.5 Veraflo is a negative-pressure wound therapy with instillation (NPWTi) system. It has two tubes, one for the instillation of solution and one for vacuum as per routine negative-pressure wound therapy (NPWT). Following some success with the use of NPWTi for infected limb salvage, we began trialling NPWTi in the management of infected breast implant pockets in 2019.
Here we document our early experience in the use of NPWTi to salvage infected breast implant-based reconstructions and present a suggested management algorithm for attempted salvage of infected breast reconstruction implant pockets.
Methods
We conducted a retrospective review of all patients identified as having NPWTi in the breast area using data obtained from electronic records at CMDHB Plastic and Reconstructive Surgery service. Regional ethics approval was obtained from the Auckland Health Research Ethics Committee (reference AH3371) and local ethics was approved by CMDHB Research Office (Registration Number 1307). The hospital electronic records were correlated with accessible paper-based medical records and surgeon specific and complication audit meeting notes to ensure complete data capture.
Data were extracted from medical records using a standard data template into an Excel workbook (Microsoft, Redmond, Washington, USA). Patient data and basic demographic information was collected from the electronic medical records, including age, ethnicity and original breast-related diagnosis. Operative information such as date, number of visits and duration of surgery were also obtained from the electronic medical records. Length of stay was determined in whole numbers from date of admission to date of discharge (inclusive).
Cellulitis was defined using the Centers for Disease Control and Prevention criteria6,7: (1) a positive aseptically obtained culture, (2) symptoms including whole breast erythema and swelling, or (3) a negative aseptically obtained culture but a physician’s diagnosis of infection for which antibiotics were prescribed.
NPTWi system (Veraflo VAC)
This system combines the computerised delivery of a solution via a pump at a prescribed time with negative-pressure wound therapy. It is set up to instil the solution for a set amount of time–in our service this is 10 minutes to soak the wound and overlying foam, which is sealed within an occlusive dressing. The machine then activates the negative pressure to a setting of 125 mmHg continuous, removing the solution from the wound for three hours (range: two to four hours). Therapy settings were able to be adjusted at the discretion of the operating surgeon if deemed necessary. This process is repeated with fresh solution over a 48-to-72-hour period until return to theatre.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, Inc, California, USA). Statistical significance was set at a p-value of < 0.05.
Results
Patient population
In 2019 CMDHB Plastic and Reconstructive Surgery service treated seven patients (eight breasts) with NPWTi for breast implant infections. All were breast reconstruction patients; no cosmetic breast augmentation patients were treated. Three were following immediate insertion of breast implant or tissue expander at the time of mastectomy; the others were following secondary surgeries. All prostheses were in the subpectoral space. Indications for insertion of breast prostheses and stage of surgery at time of infection are shown in Table 1. The average age of a patient readmitted with implant infection was 54.3 years. Patient demographics are summarised in Table 2.
Treatment course
All patients were readmitted with symptoms of implant infection—breast cellulitis, swelling, pain or fevers. Readmission was on average 15.3 days postoperatively (range 9–28 days) and the mean length of stay was 7.4 days (range 5–10 days). Each patient had between two and four operations under general anaesthetic (mean 2.5) and the NPWTi system was used for an average of 3.5 days per patient. All patients were started on intravenous (IV) antibiotics: 57 per cent on flucloxacillin (n = 4), the remaining 43 per cent on amoxicillin/clavulanic acid.
All patients with infected implants who attempted salvage (n=4) successfully retained their reconstruction. We defined this as being discharged with an expander in situ and resolution of infective symptoms. No capsular contracture or recurrent infection occurred during the follow-up period (average two years).
Proposed management algorithm
Our algorithm for attempted salvage of infected breast implants is shown in Figure 1. In summary, we aim to take the patient to theatre as soon as possible after the diagnosis of breast implant device pocket infection is made. The patient is started on empirical broad-spectrum IV antibiotics. Device explantation and cavity washout is performed using saline. Microbiology samples are sent to the laboratory. At the surgeon’s discretion, antibacterial solution may also be used for washout, similar to the solution used at the time of implantation (for example, saline with povidone-iodine plus gentamicin plus cephalosporin). If implant pocket infection is presumed during the operation, on the basis of the presence of pus or cloudy fluid, the NPWTi system is placed for a minimum of two days while awaiting culture results. The original incision is left open and the cavity is packed with foam allowing instillation (Figures 2 and 3). Postoperatively, IV antibiotic therapy is altered according to the microbial sample results.
The time taken between surgeries is used to discuss the aims and goals of therapy with the patient. Return to theatre is planned at day two to three. Intraoperatively the NPWTi system is removed, the wound is washed out again and reassessed. At this time, a breast tissue expander is placed if the operating surgeons feel the cavity is clean, with no pus or turbid fluid, and is granulating well. The wound is closed over the expander in standard fashion. Otherwise, the NPWTi system can be replaced for another three days. Further return to theatre is planned at that time. During the subsequent theatre visit, the pocket is again reassessed and an expander inserted if deemed clean. If the breast pocket is not considered clean enough for reinsertion of breast tissue expander at this time (presence of ongoing necrotic or infected tissue), attempted salvage of the breast reconstruction is abandoned. Subsequent wound care then follows with appropriate, clinically indicated management of an infected wound.
Cases
Case 1
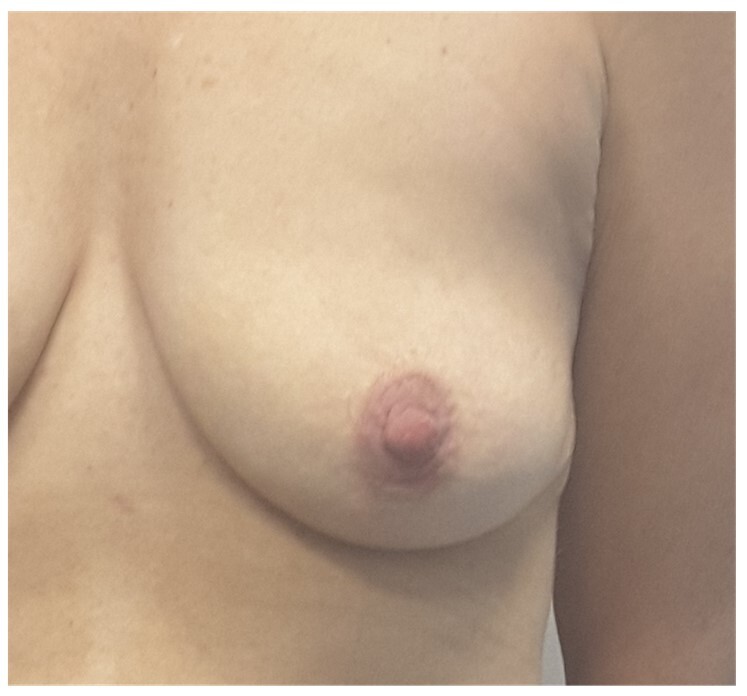
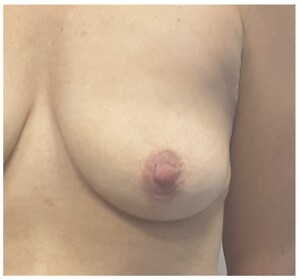
A 49-year-old woman (Figure 4) had bilateral nipple-sparing mastectomies with immediate implant and acellular dermal matrix (ADM) reconstruction in early 2019. She presented day 20 postoperatively with swelling and cellulitis of the left breast (Figure 5). She was treated with IV flucloxacillin as wound swabs had grown methicillin-sensitive Staphylococcus aureus. She proceeded for surgical washout where copious turbid fluid was seen. The pocket was washed out and NPWTi initiated. Two days later she returned to theatre where the pocket was found to be clean and granulating well. As this was early in our experience with NPWTi, the breast pocket was closed and salvage of the reconstruction was not attempted. Unfortunately, she was left with mastectomy flaps adherent to the chest wall following treatment (Figure 6). This required multiple corrective surgeries, including two rounds of fat grafting to improve the thickness and quality of the mastectomy skin flaps at five and 10 months post implant loss, followed by insertion of a breast tissue expander five months later. This was expanded in routine fashion and subsequently exchanged for a 495 cc cohesive gel anatomic breast implant 20 months after the loss of the original implant reconstruction (Figure 7).
Case 2
A 73-year-old woman had bilateral skin-sparing mastectomies with direct to implant reconstruction with SurgiMend PRS ADM and presented 13 days postoperatively with fevers and cellulitis of the right breast spreading to the left breast. This had not improved despite 24 hours of IV antibiotics so proceeded to a surgical washout under general anaesthetic. Turbid fluid was found in both breast pockets. Both implants and the ADM were removed and the breast pockets thoroughly washed and curetted. NPWTi was placed, and the patient continued with IV antibiotics. She returned to theatre 48 hours later when clean pockets were found and new expanders with 150 cc saline were inserted on each side. She remained well postoperatively and at her two- year follow-up was satisfied with her reconstructive result.
Case 3
A 52-year-old woman underwent a right mastectomy in March 2018 and had a second stage reconstruction with an expander to implant exchange in July 2019. She presented 9 days postoperatively with turbid fluid in her drain and fevers. She had 24 hours of broad-spectrum IV antibiotics then proceeded to the operating theatre for a washout of the breast pocket, where turbid fluid was seen. This was thoroughly washed and curetted and NPWTi was initiated. She returned to theatre two days later where the cavity was found to be clean and a new expander was placed with 200 cc saline. She remained well postoperatively and at her two-year follow-up was satisfied with her reconstructive result.
Case 4
A 38-year-old woman had a unilateral expander to implant exchange after mastectomy two years previously. She presented 29 days postoperatively with increased pain, breast cellulitis and increased drain output. She commenced broad-spectrum antibiotics and an ultrasound scan showed a small collection at the tip of the drain. She proceeded to the operating theatre where there was purulent fluid in the breast pocket. This was washed out and NPWTi started for six days. All foam and tubing was changed on the ward after three days. She returned to theatre for a washout after a further three days, where the breast pocket was found to be clean and an expander with 330 cc saline was inserted in the subpectoral plane. She remained well postoperatively and at her two-year follow -up was satisfied with her reconstructive result.
Discussion
In 2004, Spear and colleagues4 explored options for device salvage. Methods they described for salvaging a mildly infected device included IV antibiotics combined with either conservative wound drainage, antibiotic lavage, and any combination of capsulotomy, capsulectomy or capsule curettage with device exchange, with or without postoperative continuous antibiotic irrigation. Spear and Seruya’s follow up study2 promoting active management on infection to increase salvage rates advertised good results with a 64.4 per cent success rate, but an in-depth analysis of the cases described reveal that these were only in simple or indolent infections, as any which did not improve with antibiotics were simply explanted. Severe infections did not have surgical salvage attempted in up to 85 per cent of cases. Similarly, explantation alone was routine practice in our department, with secondary reconstruction being reconsidered six months postoperatively. We wanted to explore whether salvage of the reconstruction was possible in those with more severe infections (frank pus, severe cellulitis) who previously would not have had the option.
Our experience documents the progression of treatment aims over our first year of using NPWTi. Initially we used this system to improve the cleanliness of the explanted breast cavity following traditional explantation, debridement and wound washout (Case 1). This was performed without any expectation of salvaging the breast reconstruction. Subsequently, after we routinely saw clean, granulating wounds on removal of the NPWTi system, we discussed reinsertion of a breast implant device such as a tissue expander. As our experience progressed, we planned primary explantation, debridement and placement of the NPWTi, with a hope to replace a breast expander to salvage the reconstruction on removal of the NPWTi system as per the algorithm in Figure 1.
Use of a NPWTi system has provided an option for salvaging significantly infected breast prostheses which did not previously exist. This therapy has altered clinical care since its introduction by decreasing wound bioburden via repetitive cleaning and providing a closed sterile system.5 A randomised control trial8 comparing NPWTi to NPWT alone showed a statistically significant reduction in bacterial bioburden, reduction in wound size, improvement in granulation tissue and improvement in proliferating blood vessels and fibroblastic reaction on histological analysis. A further randomised control trial9 showed that instillation of normal saline was comparable to antimicrobial solution with regards to number of operations required, length of hospital stay and proportion of wounds closed. A 2019 International Consensus Guideline Update10 on NPWTi recommends normal saline as the first line of instillation solution, therefore saline was used as our first preference in our patient management algorithm.
Previously, NPWTi has been documented to allow complete salvage of infected breast implants. A case series of five patients published by Cheong and colleagues in 201611 described the salvage of breast pockets after implant infection using NPWTi. All patients had a surgical washout, removal of infected implants and Veraflo therapy initiated and changed every two days. On day seven, all patients had clean breast pockets and had implants of the same size reinserted therefore salvaging the reconstruction. Unfortunately, we were not able to replicate Cheong and colleagues’ results. In our experience, the use of NPWTi contracted the breast pocket to the extent that in no case were we able to safely reinsert the same sized implant as was removed, due to skin tension on the closure following contracture. Every patient in our series who had their breast reconstruction pocket salvaged did so with an expander in situ with a smaller volume than was removed. This was then expanded to the desirable size in the outpatient setting and exchanged for an implant between three to six months after salvage. To improve our outcomes in this regard we have recently changed to using a different foam called VAC Cleanse (rope shape foam) for breast pocket salvage. This is designed to contract less over the course of treatment.
Salvaging a reconstruction after breast implant infection can be a lengthy process. The patient will need a minimum of two and often three theatre visits and a lengthy inpatient stay (expected six to 10 days). The instillation fluid can feel cold to the patient and the non-portable NPWTi system is not always well tolerated as it is a large device which is not suitable for home use. On balance however, we believe the use of NPWTi to salvage a breast reconstruction at the time of infection is a worthwhile endeavour as it negates the need to begin the reconstructive process again in six to 12 months and leads to a shorter overall reconstructive journey for women.
Conclusion
Attempted salvage of an infected breast reconstruction using a NPWTi system is not a panacea for all breast implant infections. However, on balance, we feel there is benefit to using the NPWTi system for patients who can tolerate the extended inpatient stay and multiple surgeries. Using the described algorithm, we have had success with salvaging infected implants we would previously not have expected to be able to save. Therefore if the patient will tolerate the NPWTi system, it can mean continuation of their reconstructive journey with very little time delay if successful.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: July 18, 2023 AEST

.jpg)





.jpg)