Introduction
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare form of non-Hodgkin lymphoma, reported after exposure to textured breast implants. The incidence is estimated to be between 1 in 3345 cases and 1 in 11,765 cases, and exposure to alloplastic breast implants leads to 67 times higher risk of BIA-ALCL compared to primary anaplastic large cell lymphoma (ALCL).1,2 We report the shortest time frame (six and a half months) between exposure to textured breast implant and the development of BIA-ALCL.
Case
A 42-year-old premenopausal gravida 2 para 2 female presented with a palpable 25 mm left lateral breast mass with locoregional nodal disease. She was diagnosed with triple negative T2N3M0 Grade III breast carcinoma. The patient had a history of autoimmune thyroiditis, was an ex-smoker with no family history of malignancy. Genetic screening on a breast cancer gene (BRCA) plus panel was negative for germline mutation.
The patient underwent neoadjuvant chemotherapy (doxorubicin, cyclophosphamide and paclitaxel) and pembrolizumab, which ceased due to pan-hypopituitarism. Preoperative restaging confirmed the breast mass was 15 mm without nodal disease. She had a left skin-sparing mastectomy and sentinel lymph node biopsy with targeted axillary dissection. Immediate breast reconstruction with a subpectoral CPG style 311 380 cc microtextured implant and FlexHD (MTF Biologics, Edison, New Jersey, USA) acellular dermal matrix was used to achieve the most optimal aesthetic outcome.
Her final histopathology showed a complete pathological response of the tumour (Miller Payne Regression Grade 5) and negative nodes. She required two aspirations of wound seroma in outpatients within four weeks postoperatively, neither of which demonstrated bacterial growth. She completed a course of adjuvant radiotherapy to the left chest wall and nodal bases.
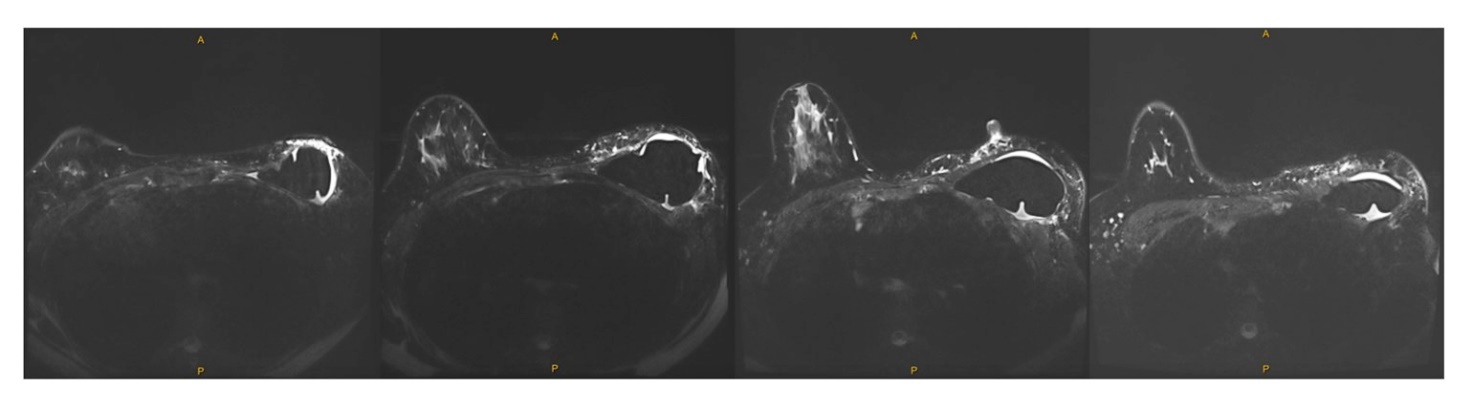
At six-month postoperative review, the patient reported aching in the left breast associated with asymmetrical swelling. Mammography and ultrasound demonstrated a moderate volume of peri-implant fluid. Bilateral breast magnetic resonance imaging (MRI) showed a left breast implant in situ with moderate pericapsular fluid (Figure 1). Fine needle aspiration was performed, and cytology examination showed a mixed chronic inflammatory cell population with CD30-positive immunoreactivity of atypical cells (Figure 2). Flow cytometry showed an abnormal T-cell population (28% of lymphocytes) with CD45+, CD4++, CD3−, CD7−, CD5− phenotype. The case was reviewed by a second independent pathologist and the multi-disciplinary tumour board meeting, which confirmed the pathological findings.
The time between implant placement and BIA-ALCL was six months and 16 days. Subsequent staging was clear. She underwent a staged procedure to remove the breast implant and en bloc capsulectomy. There was no evidence of BIA-ALCL within the capsular tissue on histopathological assessment consistent with early-stage disease (Stage 1A, T1 effusion only Nx Mx).
Discussion
Breast implant-associated anaplastic large cell lymphoma is a distinct variant of ALCL. While the molecular pathogenesis of BIA-ALCL is still poorly established, there is a strong association with textured breast implants (zero reported cases in smooth implants). A chronic inflammatory response to implants may occur, with sensitisation of T-lymphocytes to capsule-related biomaterial3 leading to IL-6 and IL-10 mediated activation of the Janus kinase signal transducer and activator of transcription (JAK-STAT) signalling pathway and dysregulated cell proliferation.3 An association between subclinical low-grade infection and BIA-ALCL may occur, with larger numbers of gram-negative bacteria isolated in samples associated with BIA-ALCL, and higher rates of coagulase-negative bacteria present in the biofilm of textured implants compared to smooth implants.3 Key pathogenic pathways may be dysregulation of the JAK-STAT pathway, abnormal p53 function and MYC amplification. The JAK3 germline V7221I variant is thought to be a genetic predisposing factor for BIA-ALCL,4 and two cases have been reported in patients with TP53 germline mutations.2,5
Clinical symptoms include seroma, pain, capsular contracture, lymphadenopathy and a breast mass.1 The mean onset ranges from seven to 10 years post-exposure to implants (two years is the earliest reported case so far).2 Current international guidelines recommend triple assessment—clinical examination, mammography and ultrasound (or MRI)—for a patient with breast implants presenting with these symptoms. Patients with late-onset or persistent peri-implant seromas with breast implants should undergo aspiration for cytology.2,5 Cytology shows medium to large atypical lymphoid cells with irregular kidney or horseshoe-shaped multinucleate cells and neoplastic cells stain CD4 and CD30 positive, and CD3, CD5 and CD7 negative (loss of T-cell phenotype).1,5
Following staging and discussion at an oncological multidisciplinary meeting, the mainstay of treatment is surgery with complete en bloc resection of the capsule and breast implant. The management of the contralateral breast implant remains controversial. Typically BIA-ALCL is an indolent disease with favourable outcomes. However, advanced-stage patients may have an aggressive course, with nodal and distant metastases present at diagnosis increasing mortality risk,1,2 and adjuvant systemic therapy required.
The short interval between implant exposure and the development of BIA-ALCL in this case is unusual. Flow cytometry was highly sensitive and identified an abnormal clone early in the development of BIA-ALCL before tumour nodules or capsular invasion developed.
This case is complicated by the exposure to pembrolizumab, a monoclonal antibody that stimulates T-cell-mediated tumour cell death.6 Given the proposed pathophysiology of BIA-ALCL is a chronic inflammatory process, further research to explore if there is a link between pembrolizumab and BIA-ALCL is required. There are no cases to date describing BIA-ALCL arising within pembrolizumab treatment, only a case of BIA-ALCL developing with adalimumab therapy.7 Pembrolizumab has been associated with rare lymphomas, with a USA Food and Drug Administration review showing an 0.02 per cent incidence of T-cell lymphoma in patients receiving immune-checkpoint inhibitors (pembrolizumab, ipilimumab and nivolumab).8–10 Retrospective analysis of some pre-treatment samples showed the existence of the neoplastic clone at low frequency, and the use of immunotherapy may have hastened the early onset of BIA-ALCL. Furthermore, pembrolizumab has been shown in some patients to markedly elevate pro-inflammatory cytokines, including IL-6 and IL-10, which may be associated with BIA-ALCL pathogenesis. Further studies to determine if there is a link between immunotherapy agents and BIA-ALCL are needed.
Conclusion
As the rates of alloplastic breast augmentation and reconstruction increase, the incidence of BIA-ALCL may increase too. This case prompts surgeons to maintain a heightened suspicion of BIA-ALCL in patients with persistent peri-implant fluid regardless of the time post-implant surgery, and an awareness of possible effects of adjuvant therapies in early presentation of these rare lymphomas.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: May 22, 2023 AEST