Introduction
Sweet syndrome (SS) has been widely described in the dermatological, histological, immunological and surgical literature. Multiple clinical and histological variants have been examined. Neutrophilic dermatosis of the dorsal hands (NDDH) is one of the variants and presents with indurated, painful, erythematous plaques mixed with ulcers and pustules. We present a case of NDDH occurring after the patient starting vaping, with the patient’s written consent and cooperation with publication.
Case
A 64-year-old, right-hand-dominant, retired female presented to our hospital with bilateral hand ulcerated lesions. She reported that the lesions started as red patches on her right hand that increased in size, blistered and changed colour to purple, and eventually progressed to her left hand. She reported quitting smoking tobacco and starting vaping nicotine three weeks prior. She initially held the vape using her dominant right hand between her thumb and index finger, but after developing blisters she switched to her left hand, which also developed largely symmetrical lesions. She reported that the vape got hot during use but not hot enough to cause thermal burns. She denied any trauma, contact with livestock or autoimmune disease. There was no improvement from oral cefalexin prescribed by her general practitioner.
The patient’s medical history included type 2 diabetes and hypertension, for which she took metformin and metoprolol.
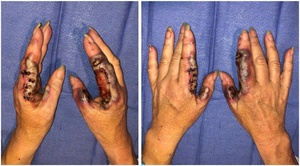
Examination revealed bilateral hands with bluish-purple pseudo-bullous nodules with largely symmetrical blistering over radial borders of index fingers and ulnar borders of thumbs (Figure 1). Both hands were tender on palpation. The affected digits were moderately swollen, compared to the remainder of the hands and non-involved digits. The patient had reduced flexion due to pain and normal active extension. There was no evidence of flexor tenosynovitis or deep space collection. Her sensation and capillary refill were normal. She did not have any other rash or lymphadenopathy and was afebrile.
The patient’s C-reactive protein was raised at 16, the white cell count was mildly raised at 11.5 (neutrophils 8.2; lymphocytes 3.1) and the X-ray was normal. The tissue culture and swabs were negative for bacterial, viral, fungal or mycobacterial infection.
The patient was admitted for intravenous antibiotics and minimal debridement with biopsies in the operating theatre (Figure 2). Several punch biopsies were taken from the edge of the lesion and sent for histopathology, microscopy and culture (including Mycobacterium ulcerans). Sloughy epidermis was debrided with wet gauze and wounds were washed with saline.
The histopathology showed quite prominent inflammatory infiltrate in the dermis with quite a few polymorphs, consistent with neutrophilic dermatoses. No fungi, viral inclusions or malignancy were seen.
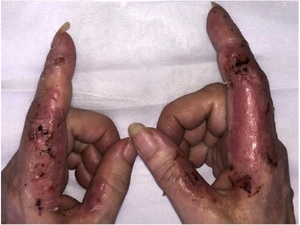
Our patient was reviewed by dermatology and following all the investigations a diagnosis of acute NDDH (a variant of SS) was made. The patient was started on topical methylprednisolone aceponate 0.1 per cent ointment (Advantan) once daily as well as oral prednisolone 25 mg once daily (weaning by 5 mg every seven days, then stopping). The patient was then discharged to dermatology for future management. She made a remarkable recovery without any aggressive surgical debridement and skin grafting, allowing wounds to heal by secondary intent as seen during the clinic review almost two weeks after starting the treatment (Figure 3).
Discussion
Sweet syndrome was first described as an acute febrile neutrophilic dermatosis by Dr Robert Sweet in 1964, who observed ‘raised painful plaques on the limbs, face and neck’ of eight women associated with leukocytosis and ‘histologically a dense dermal infiltration with mature neutrophil polymorphs’.1
It is a rare phenomenon and its true incidence in the general population is yet to be established. Due to its rarity, current literature includes predominantly case reports and case series. It usually presents in the sixth decade of life, but does not spare children. To date, there have not been any case reports of SS associated with vaping.
The pathogenesis of SS is multifactorial. It is a product of external physical insults (chemicals causing pathergy), immune dysregulation (malignancy or autoimmune disease) and genetics.2 There are three distinct subtypes based on the aetiology—idiopathic, malignancy-associated and drug-induced.3 Literature describes several iatrogenic triggers of SS including certain drugs such as granulocyte-colony stimulating factor (G-CSF), FLT3 inhibitors (leukaemia treatment), all-trans retinoic acid, clopidogrel, dapagliflozin, antibiotics, nonsteroidal anti-inflammatory drugs, COVID-19 vaccines and illicit drugs.4 Pathergy, photoinduction and radiotherapy have also been implicated in pathogenesis.4
None of the previous studies reported vaping-induced SS. Vaping contains between 60 and 120 compounds on average in variable concentrations.5 Many of those compounds are toxic substances such as heavy metals, volatile organic compounds, propylene glycol and harmful solvent by-products (eg, formaldehyde), that can promote inflammation and allergic reaction.6 Moreover, Lalla and colleagues noted that the higher the concentration of propylene glycol as a solvent, the more intense the reaction in both allergic and irritant contact dermatitis.7
Contact allergy has been reported with electronic cigarettes in a person with a known nickel allergy.8 Urticarial reactions have also been associated with cadmium contamination after vaping.9
Besides allergic reactions, there have been many other cutaneous manifestations among vape users described in the literature, including burns, nicotine stomatitis, hyperplastic candidiasis, black hairy tongue, surgical skin flap necrosis and discoid lupus erythematosus associated with electronic cigarette use.10,11 The authors describe upper lip and palate involvement predominantly, which they attribute to the Koebner phenomenon, which is the appearance of new skin lesions as a direct result of trauma of previously unaffected skin. This phenomenon represents an induction of inflammatory skin lesions following trauma, which may explain SS in our patient when she switched holding the vape from her right hand to her left and subsequently developed new lesions in her previously unaffected left hand. This further explains the formation of SS lesions at sites of radiation therapy, surgery, burns and tattoos.12 Gloor and colleagues described a case of NDDH involving bilateral hands similar to our patient following the use of garden shears.13 The lesions appeared at the first web spaces following mechanical trauma during gardening with shears while wearing gloves. The lesions did not respond to oral antibiotics and following a skin biopsy the patient was started on oral and topical steroids with complete resolution of the lesion after 10 days. This concept of pathergy may explain why low-grade heat exposure from holding the vaping device, as well as toxic substances, may also be implicated in the pathophysiology of SS. We postulate that it was the low-grade heat exposure that initiated SS in our patient, because of the distribution of lesions on both hands in a staged fashion as she switched holding the device and the unaffected lips and palate. However, the gaps in the current literature led to the incomplete nature of our understanding of the pathogenesis of this disease.
Moreover, Cervellati and colleagues analysed the effect of vaping on human keratinocytes.14 The authors concluded that a panel of pro-inflammatory cytokines (PDGF-BB, IL-8, IL-10, IL-12, IL-17, GM-CSF, G-CSF, IFN-γ, TNF-α, VEGF) and chemokines are increasingly released by keratinocytes after vaping. These cytokines cause neutrophil differentiation, maturation and activation, which infiltrate the superficial and mid-dermis, causing oedema and epidermal sloughing off in the form of pustular, bullous and pseudo-bullous lesions. Bullae represent fluid-filled blisters within the epidermis, whereas pseudo-bullae are due to acantholysis—separation of the epidermis as a result of the dissolution of intercellular connections between keratinocytes. Histologically, the bluish-purple appearance is due to erythrocyte extravasation into the dermis.2 The appearance may cause misdiagnosis of infection leading to extensive surgical debridement and propagation of lesions.13 Steroids remain the drug of choice in the management of SS. Both topical and systemic use can provide excellent responses, as is seen in Figure 3 after a week of therapy.
Conclusion
Electronic cigarettes were developed as an alternative to tobacco smoking. While their use has steadily risen throughout the world, so have the adverse reactions, including allergic reactions, burns, lupus and oral mucosal lesions.
We postulate that the cause of SS in our patient is vaping and we believe more cases will be observed worldwide as vaping becomes a more popular alternative to cigarette smoking. It is worth noting that Australia has mainly banned vaping and New Zealand has highly regulated its use compared to the rest of the world.
Patient consent
Patients/guardians have given informed consent to the publication of images and/or data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding declaration
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: January 14, 2024 AEST; February 4, 2024 AEST