Introduction
Perineal urethrostomy (PU) is a valuable technique in the management of anterior urethral strictures. It is indicated in situations where urethroplasty is unable to be performed; as a salvage for failed urethroplasty, or as the first step of a staged urethroplasty.1 It is also utilised following penectomy or urethrectomy to avoid the need for a permanent suprapubic catheter.2 Perineal urethrostomy allows patients to maintain continent voiding, and has been shown to maintain patient quality of life.3
Traditional operative techniques, as described by Johanson and Blandy, incorporate perineal and scrotal skin flaps to allow the urethra to be mobilised without excessive tension, minimising rates of stenosis.4,5
Failure of PU has been defined as any patient requiring post-operative instrumentation, and the reported rate of failure ranges from 21.6–30.0 per cent.3,6–8
Multiple factors can contribute to failure of perineal urethrostomies. These include recurrence of the underlying pathology (such as urethral cancer or lichen sclerosus), inadequate debridement of involved proximal urethra, wound infection, or excessive tension at the anastomosis.9 Radiotherapy,7 prior failed urethroplasty, and a traumatic or infective stricture aetiology3 have been shown to increase risk of PU failure.
Currently used salvage techniques for failed PU include repeated local random-pattern flaps10,11 and buccal mucosal12 or split-thickness skin grafts,9 with only modest success being reported. The only alternative after failed revision PU is urinary diversion, such as supra-pubic catheterisation or ileal conduit formation.9
Our aim was to develop a technique that enabled creation of the urethrostomy to an adequate calibre using vascularised tissue based on known regional perforator anatomy, sufficient to permit tension-free closure, in cases that would be high risk or unsuitable for traditional PU methods.
Methods
Following general anaesthesia, the patient is placed in the lithotomy position. Perforating vessels of the internal pudendal system are identified using hand-held Doppler ultrasound. Prophylactic intravenous antibiotics are administered prior to incision, and the urethra is debrided to healthy viable tissue. The proximal urethra is spatulated and absorbable stay sutures are inserted.
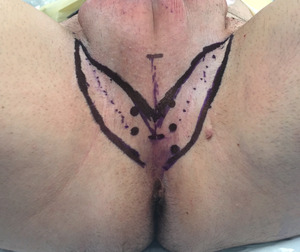
The distance from the perineal skin to the urethra is measured, and the lotus petal flap is marked incorporating the previously identified perforating vessels (Figure 1).
The initial incision is made and then the flap is raised in a supra-fascial plane from distal to proximal in the manner previously described, with identification and dissection of the perforating vessels (Figure 2).
The skin paddle is completely islanded if desired or may be left with a posterior skin bridge intact (to help direct the flow of urine). If islanded, the flap may be completely tubularised to form the full circumference of a neo-urethra, in cases where there is extreme shortage of native urethral length (Figure 3). The donor site is closed in layers over a suction drain, and the flap is inset to form the urethrostomy using absorbable sutures (Figure 4). A Foley catheter is inserted, and remains in place for five days postoperatively. Drains are usually removed on the first postoperative day. There are no specific positional nursing requirements.
Results
To date, this technique has been used in three patients through our institution. Their cases are summarised in Table 1. All patients have demonstrated continent voiding with a minimum follow-up of 22 months, with no requirement for instrumentation or revision. All patients were assessed with flexible cystoscopy between six and nine months post-operatively to confirm patency.
Discussion
Soft tissue defects of the perineum have long represented a complex challenge for the reconstructive surgeon. Multiple reconstructive modalities have been described for wounds of this region, including regional myocutaneous flaps and local random-pattern or axial skin flaps.13 More recent developments have included perforator-based flaps.14
The lotus-petal flap was first described in 1996 for use in the reconstruction of vulvo-vaginal defects.15 Since that initial description, it has been used in a range of indications for wounds of the perineum, including scrotal and perianal defects.16,17 In their initial description, Yii and Niranjan advocated elevating the flap with the deep fascia and identifying the perforating vessels. Warrier and colleagues have described an adipocutaneous modification of the flap that does not involve identification of the perforators.18
A related flap, the pudendal thigh flap, has previously been used in the reconstruction of traumatic posterior urethral strictures.19 However, to our knowledge this is the first report of local perforator-based flaps for perineal urethrostomy.
Previous authors have described multiple techniques to address failure of perineal urethrostomies. Many patients undergo repeated dilatations of the stricture, but this technique does not address the underlying pathology and is usually only a temporary solution. Revision PU with buccal mucosal grafts have been described, but this is limited by the availability of donor tissue.12
The ‘7-flap’ technique described by French and colleagues is a recent technique aimed at minimising failure in PU.10 It utilises a local random-pattern flap that presents several advantages over the traditional perineal and scrotal flaps of the Johanson and Blandy techniques, including the ability to design the skin flap once the defect has already been created. However, given its dimensions and the random nature of its vascular supply, there remains a significant risk of wound tension and flap necrosis, particularly in high-risk patients. It also relies on local tissue that may not be free of the underlying disease process, such as radiotherapy damage or lichen sclerosus.
Lumen and colleagues have described the use of split-thickness skin grafts (SSG) for revision PU in a single case of urethral SCC.9 However, most revision PU cases involve poorly vascularised wound beds and fibrosis following previous surgery, infection or irradiation. Secondary contracture of SSGs can lead to a total failure of the PU due to stenosis.
The lotus petal flap confers numerous advantages over the existing reconstructive options. It represents a thin, pliable flap with a reliable vascular pedicle over a rich anastomotic network that lies outside the ‘zone of injury’ of disease. It is possible to orient the flap donor site in a number of ways, allowing reliable modification to suit the requirements of a particular defect. The donor site is well tolerated. Bilateral flaps can be reliably raised in cases where there is a need for additional soft tissue. In cases where insufficient proximal urethral stump exists for traditional PU, the lotus petal flap technique offers a solution by bridging the urethral defect and the perineal skin with a vascularised tube. This is especially important in urethral cancer cases where a very proximal amputation is required for oncological clearance, and which would usually mandate more proximal urinary diversion.
The lotus petal design allows for both partial and total circumferential inset for formation of the PU. The latter is achieved either by utilising two (bilateral) flaps or a completely islanded and tubularised single flap (as demonstrated in case three). There are myriad alternative configurations possible, including separate flaps for the PU and perineal resurfacing when required.
There are several potential disadvantages to the use of the lotus petal flap in PU. Perforator flap dissection can be technically challenging and should only be performed by an experienced reconstructive surgeon. The vascular pedicle can be at risk from injury by the underlying disease process, particularly in cases of trauma. Finally, the lotus petal flap contains hair-bearing skin. However, this is a factor common to all locoregional cutaneous flap options and has not proven problematic in our case series thus far. In our series we have used perforator flaps based on the pudendal artery system. However, a number of perforator and axial-pattern flaps have been described in the perineal region and could theoretically be applied to this technique. Our aim is to apply reconstructive surgical principles to a common urological problem to improve patient outcomes.
We have used the lotus petal flap in primary PU formation, as well as secondary cases after traditional flap techniques have failed and it has subsequently been used successfully at several high-volume urogenital reconstructive centres worldwide. The technique should be considered in patients undergoing perineal urethrostomy that are at high risk for failure, including revision cases, those that are anticipated to require larger amounts of tissue to prevent excessive wound tension (such as obese patients or those requiring more extensive proximal urethral resection), and patients with local or systemic factors predisposing to wound healing problems (such as prior irradiation, diabetics, smokers, or immunosuppressed patients).
We do not suggest that this technique should be applied to all patients undergoing PU; in the majority of cases, traditional techniques are safe and reliable. Our technique renders the procedure more reliable in high-risk patients, as well as broadening the indications to include patients that traditionally would not be considered for PU.
Conclusion
Utilisation of lotus petal flaps in high-risk cases of perineal urethrostomy will lead to significant improvements in patient outcomes. The availability of larger amounts of soft tissue coverage will obviate the need for compromise on either resection of involved urethra, or calibre and inset of urethrostomy. This will subsequently minimise the rates of failure, and reduce the requirement for urinary diversion procedures.
Acknowledgements
This work has previously been presented at the following meetings: Société Internationale d’Urologie (SIU) 35th Congress, Melbourne, Australia, October 2015; Societe Internationale d’Urologie (SIU) 36th Congress, Buenos Aires, Argentina, October 2016; 7th International Meeting of Reconstructive Urology (IMORU), Hamburg, Germany, March 2016; Genitourinary Reconstruction (GU-RECON) Live International Symposium, Pune, India, March 2016; 17th International Course on Perforator Flaps, Sydney, Australia, November 2016.
Disclosure
The authors have no conflicts of interest to disclose.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Revised: November 26, 2017